Original paper: Microfluidic study of retention and elimination of abnormal red blood cells by human spleen with implications for sickle cell disease
Content review: Arthur Michaut
Style review: Arthur Michaut
Though they may not realise it, anyone who’s taken the subway at rush hour knows how a red blood cell feels passing through the human spleen. Almost home now, just need to get through the gates; but wait, someone’s ticket isn’t working, the crowd is starting to push, the gates are getting jammed… Maybe you should have called a cab.

Much like turnstiles validating your ticket, the human spleen contains narrow slits that red blood cells must squeeze through to prove they are fit to navigate tight blood vessels while carrying oxygen around the body. The spleen is tasked with ensuring our blood contains the right amount of red blood cells and removing any cells that aren’t in tip-top shape. Shape, however, can be a problem the spleen can’t handle.
People with sickle cell disease produce “sickle” shaped red blood cells due to an inherited genetic mutation. Sickle cell disease patients can experience episodes of acute splenic sequestration, or “spleen crisis” where these misshapen cells clog the slits of the spleen, depriving it of oxygen and leading to swelling that can become life-threatening. Because this process is difficult to observe and monitor in the body, there is little understanding of how and why a spleen crisis might occur. When a spleen crisis occurs, an urgent blood transfusion is needed to treat the problem, however, in some cases, it becomes necessary to surgically remove the spleen.
A group of scientists spanning the USA, France, and Singapore have teamed up to understand how sickle cell disease can become life-threatening. Their device, a silicone model spleen, could be used to predict complications of the disease as well as develop new treatments.
Using a soft material classically used in microfabrication, named PDMS, to cast a mold with slits similar to those in the human spleen (Figures 1 & 2i), researchers were able to directly observe the processes that lead to spleen crises. The group analysed flows of red blood cells from both healthy and sickle cell disease patients through the silicone spleen, simulating splenic blood flow rate and oxygen levels, and measured retention of cells in the slits of the device.

After one minute of blood flow, red blood cells from sickle cell disease patients blocked more than double the number of slits as healthy red blood cells (Figures 3i & 3ii), demonstrating how the spleen can become blocked and swollen in sickle cell disease patients. When the silicone spleen was deprived of oxygen, as occurs during a spleen crisis, red blood cells from sickle cell disease patients became stiffer, more viscous, and more frequently sickled (Figure 3iii). Under this condition, sickled red blood cells completely blocked all of the slits. Upon reintroducing oxygen into the system, many cells return to their normal shape and blockages begin to open up again within seconds (Figures 3iv & 4).




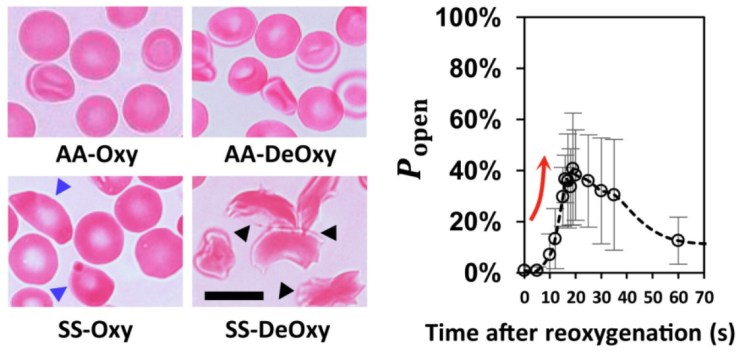
The results uncover a viscous cycle leading to spleen crises in sickle cell disease patients. Sickled cells cause blockages in the spleen, which deprives the spleen of oxygen, which in turn causes more cells to become sickled. Additionally, it was shown that the addition of oxygen caused cells to “unsickle” and rapidly cleared blockages in the slits, revealing why immediate blood transfusion can alleviate spleen crises. The researchers speculate that a sudden burst of oxygen-rich red blood cells via transfusion has the same effect as when they reintroduced oxygen to their blocked system.
Researchers believe the spleen-on-a-chip could become a new tool to allow sickle cell disease patients to monitor their condition and allow for early diagnosis of spleen crises, as well as providing a testing ground for new treatments against the disease. Safe travels red blood cells!
Disclosure: The author declares no competing interest.


