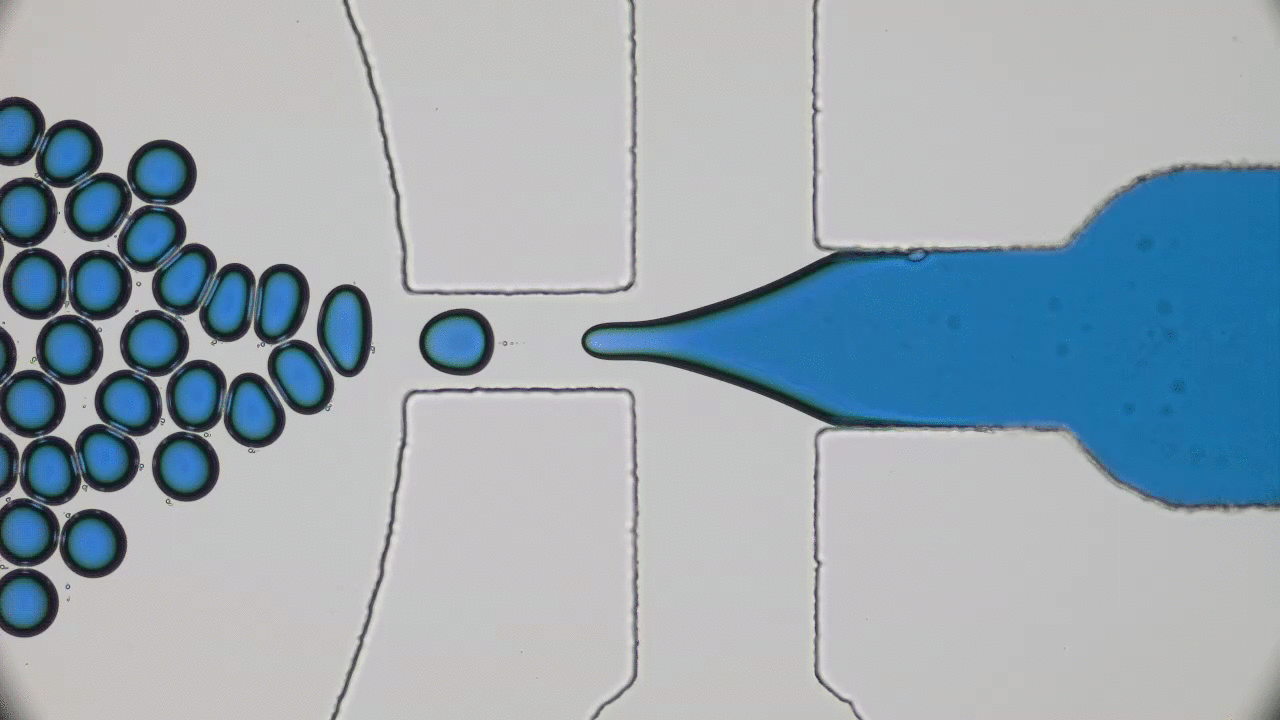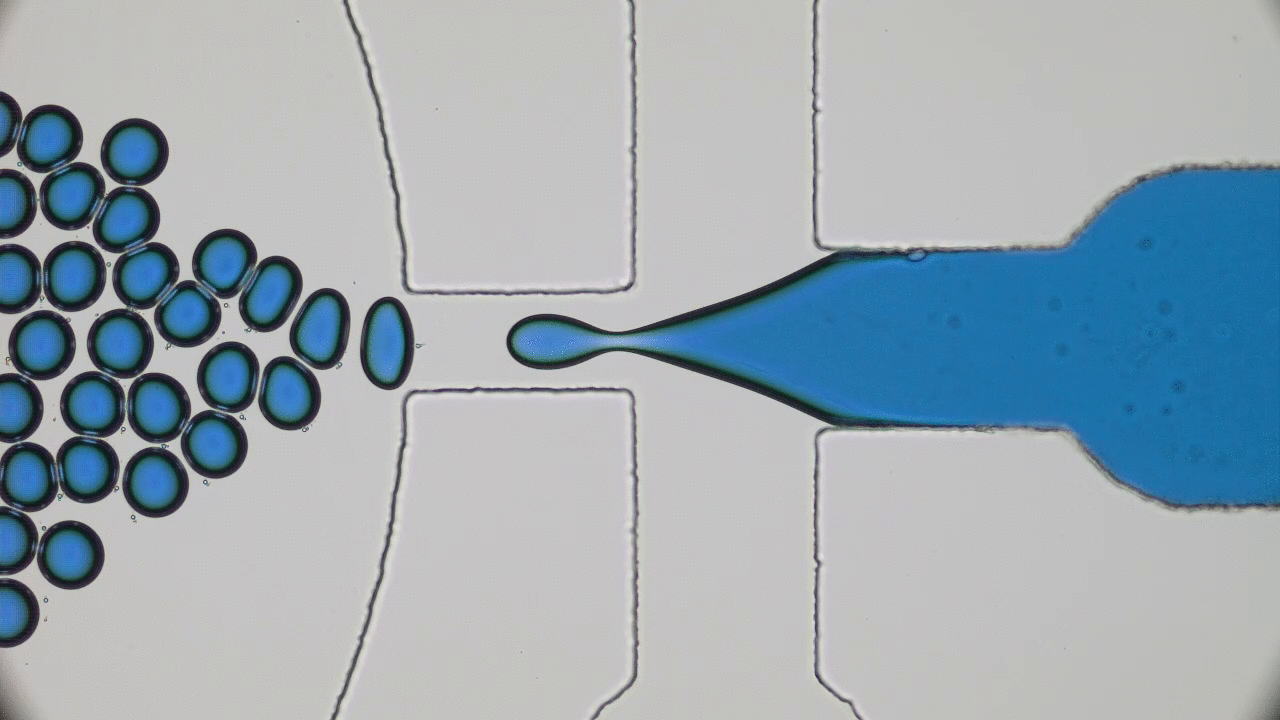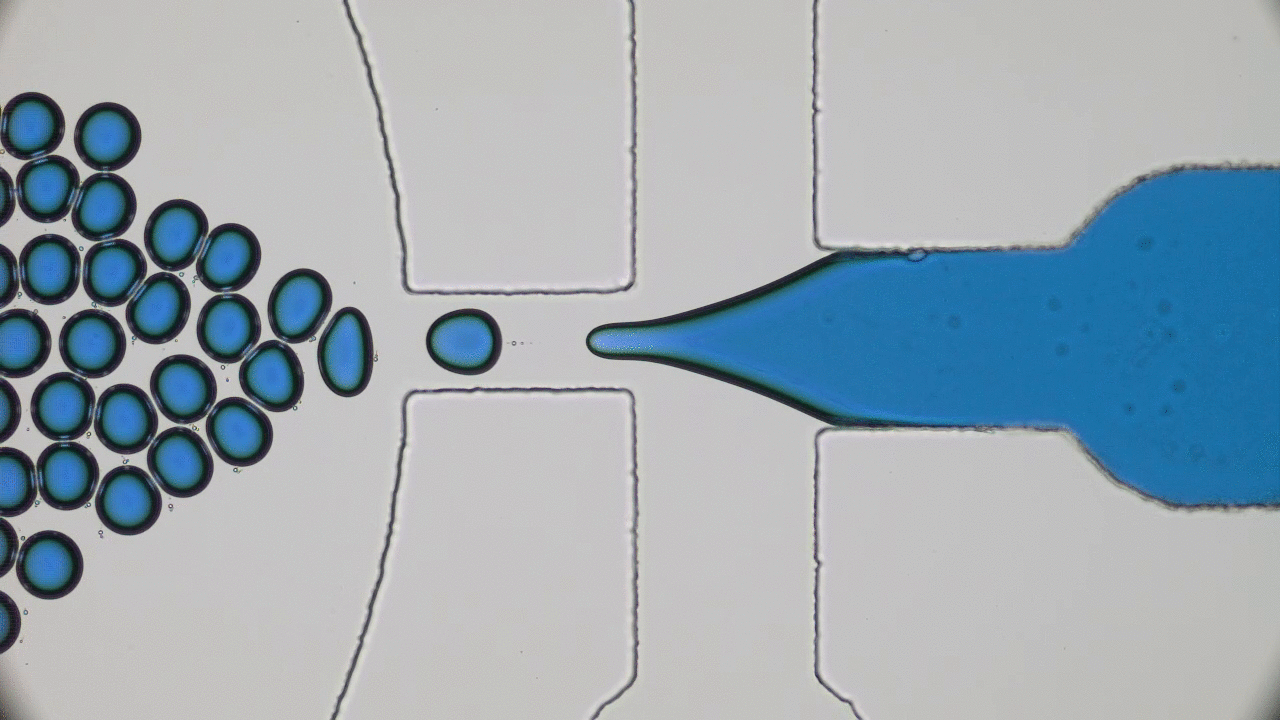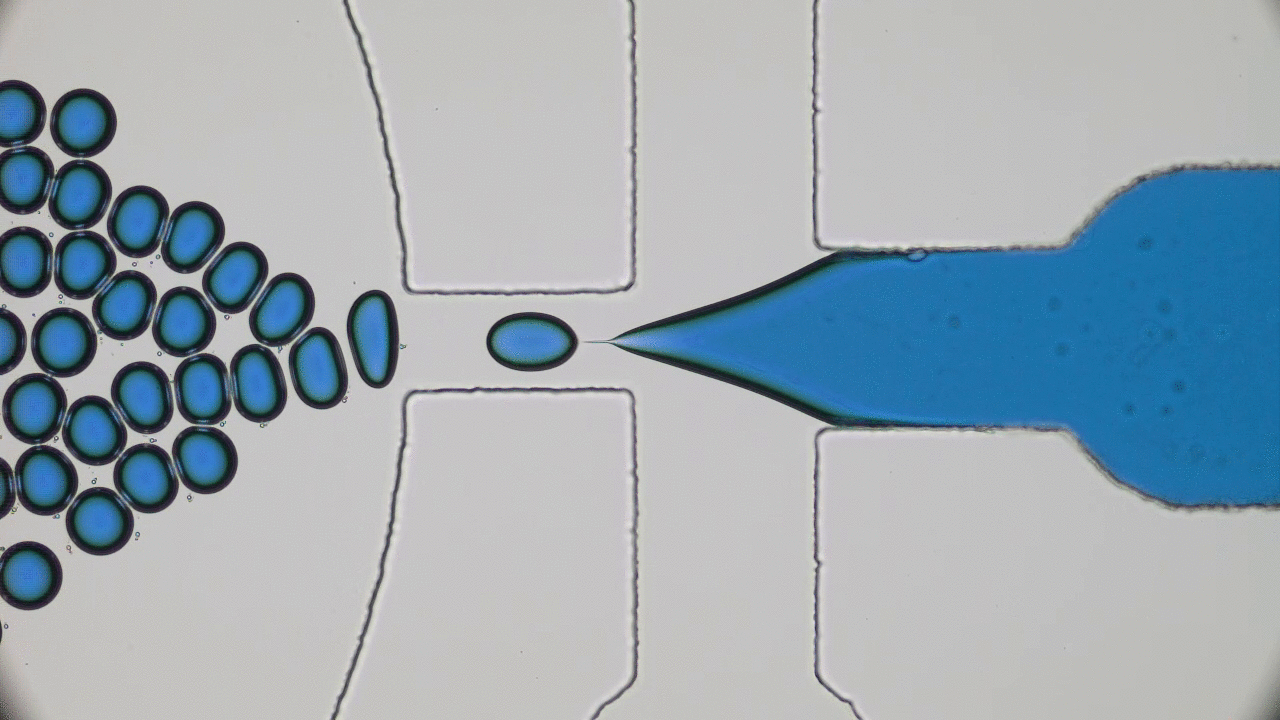
Many living creatures, such as birds, sheep, and fish, make coherent flocks or swarms. Flocking animals travel together, coordinating their speed and turns in an often visually striking manner. This can have benefits for the animals – flocking birds can use aerodynamics to fly more efficiently, sheep can move together as a group to evade predators, and fish can use collective sensing to find preferred locations in their environment. Flocks emerge in biological systems because animals try to follow their neighbors.
But how about non-living things? Can they spontaneously form swarms without any biological motive?