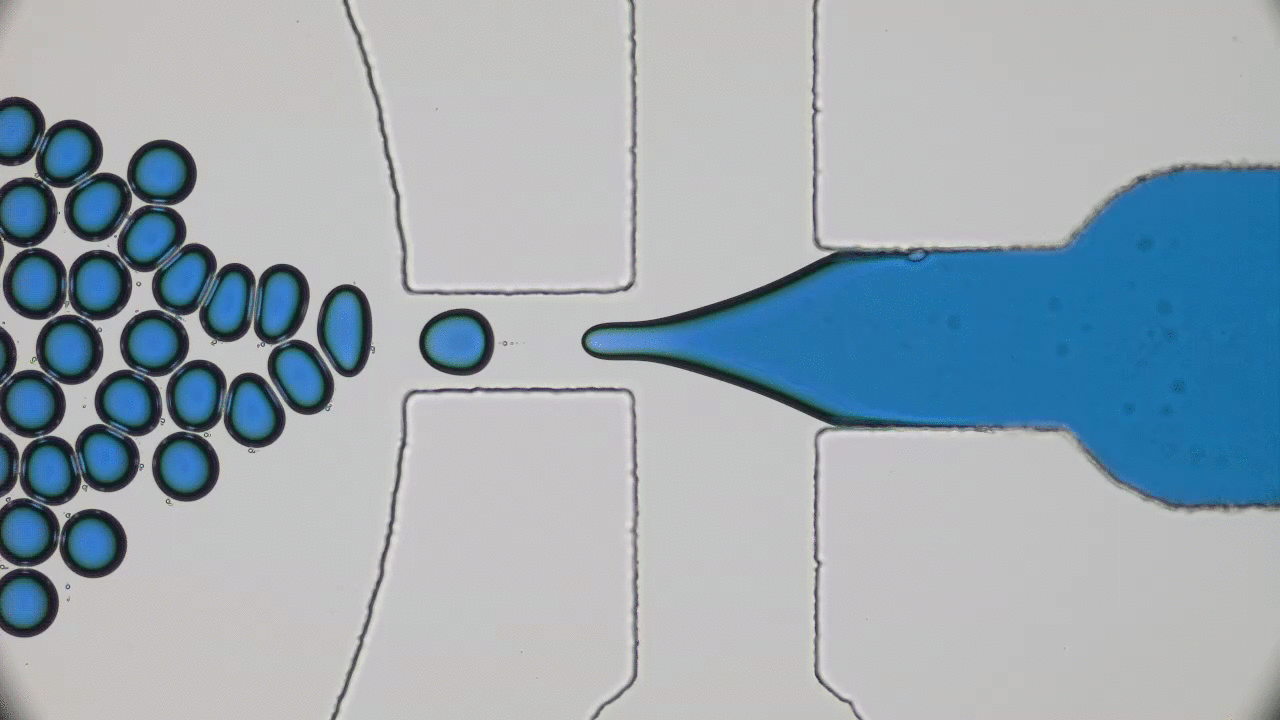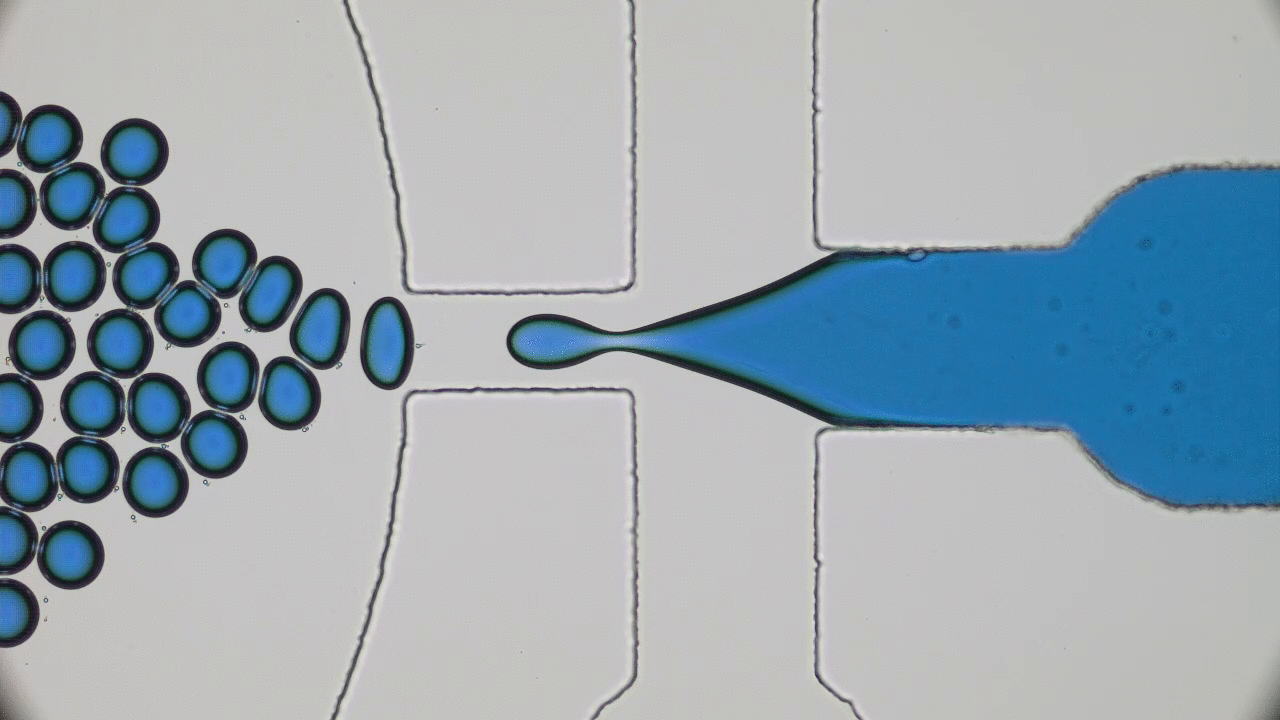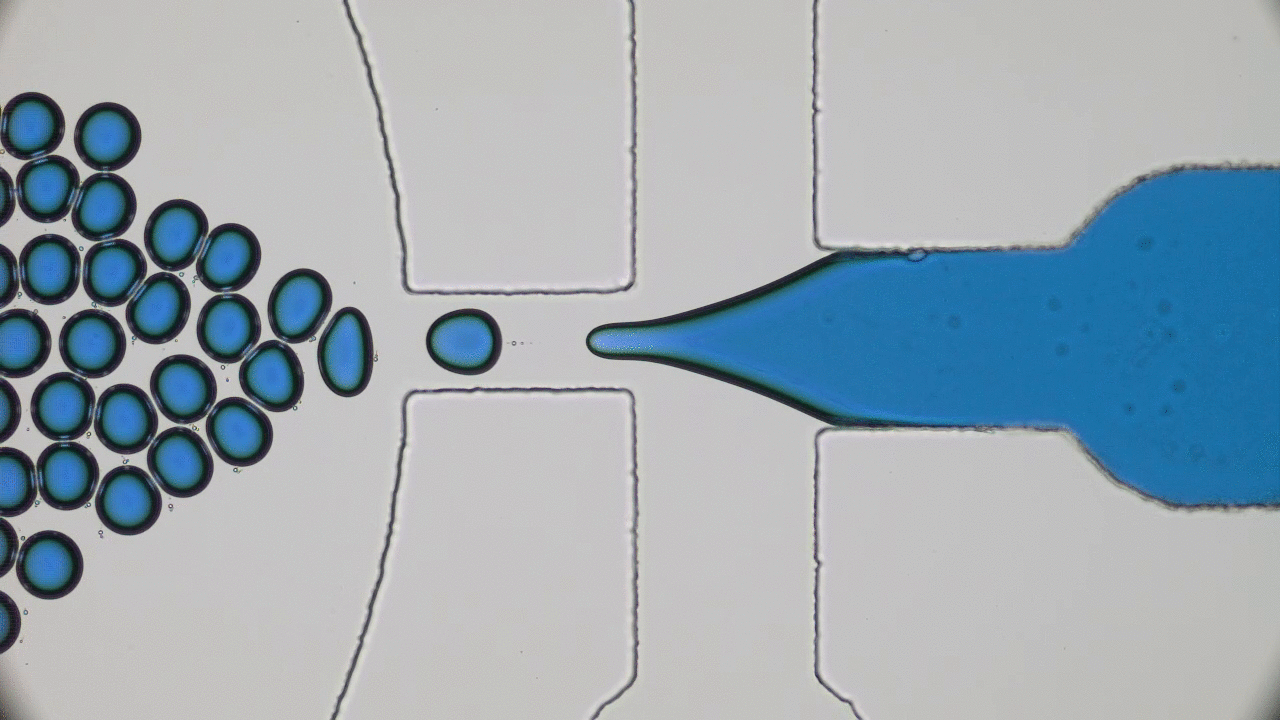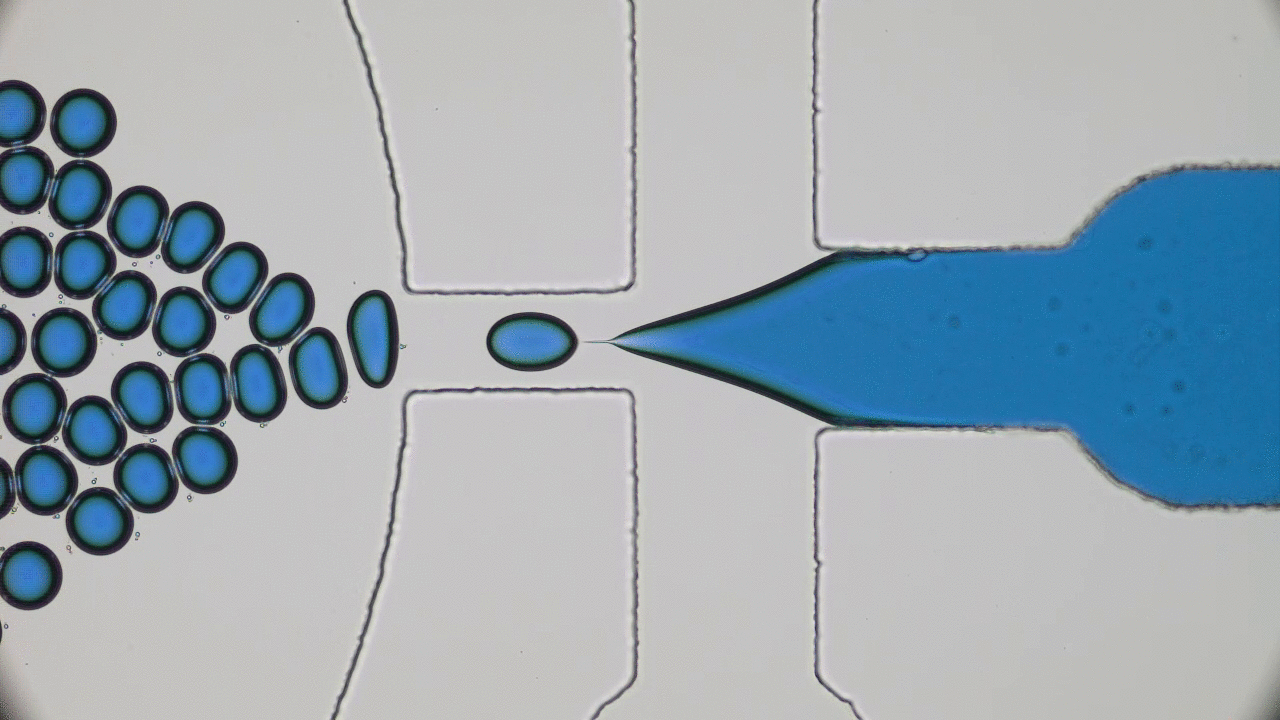
In my previous post on soft nanoparticles, you were introduced to polymer-based nanoparticles that could be used in biomedical applications, one of which is cancer therapy. These nanoparticles have a range of useful properties for cancer treatments, including their spherical shape and small size (~100 nm), both of which are similar to exosomes, small globules that are used in nature for transferring proteins between cells. Since cells naturally absorb exosomes, artificial particles with this size and shape should also be easy for cells to absorb, which means these particles could be used to deliver drugs into cells. While this idea sounds promising, it hasn’t worked out in practice — when drug-loaded polymer-based nanoparticles were injected into a tumor, subsequent tests showed that less than 1% of the injected dose entered the cancer cells. Since these particles were the correct size and shape, why didn’t they get inside the target cells?
Soft nanoparticles: when polymers meet soap
For more than four decades, scientists have been investigating the properties of small objects dispersed in solutions. Some of these objects – produced in laboratories – are the so called soft nanoparticles. The name soft comes from the fact that these particles are partly solid and partly liquid. One of the scientists’ aims is to design nanoparticles that will be used as carriers of medical compounds (like drugs, DNA segments, and enzymes). The nanoparticles’ role will be to protect this cargo from partial degradation through the human body until reaching the specific target cells where the nanoparticles’ structure will break up and the useful compounds will be released. This technology will allow for disease treatments using smaller amounts of drugs, which will mean fewer side effects for the patients.