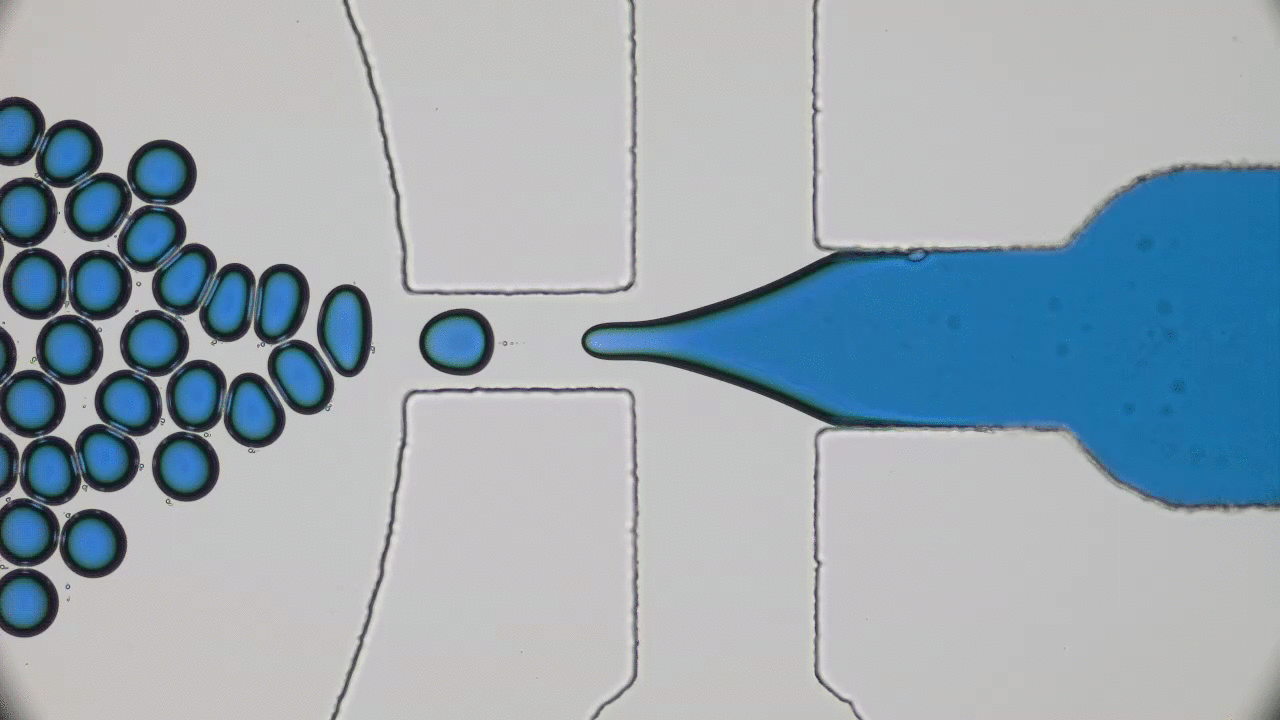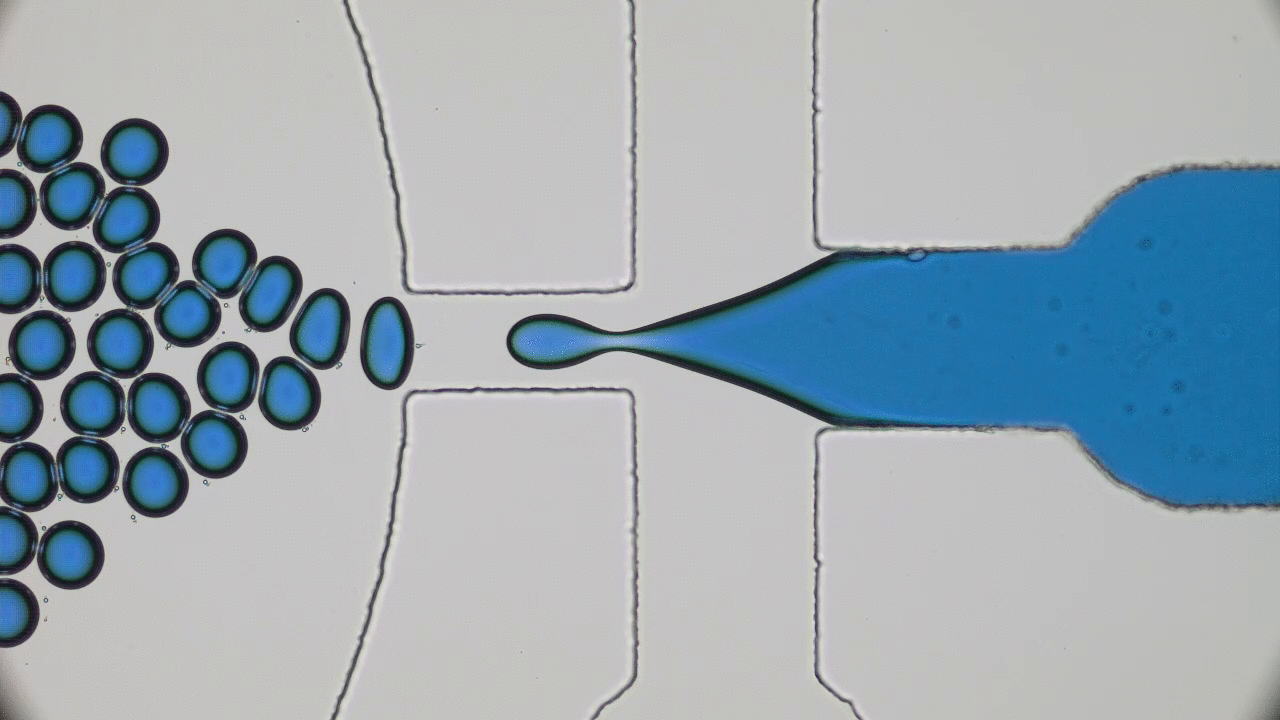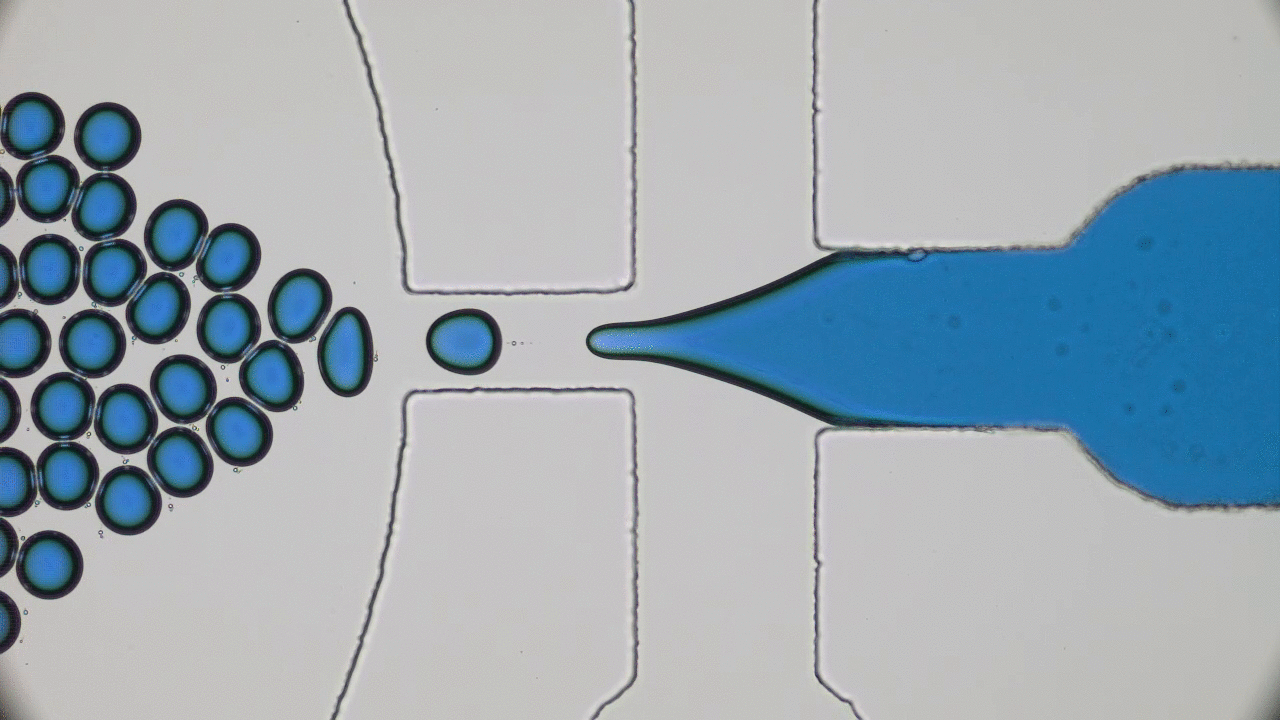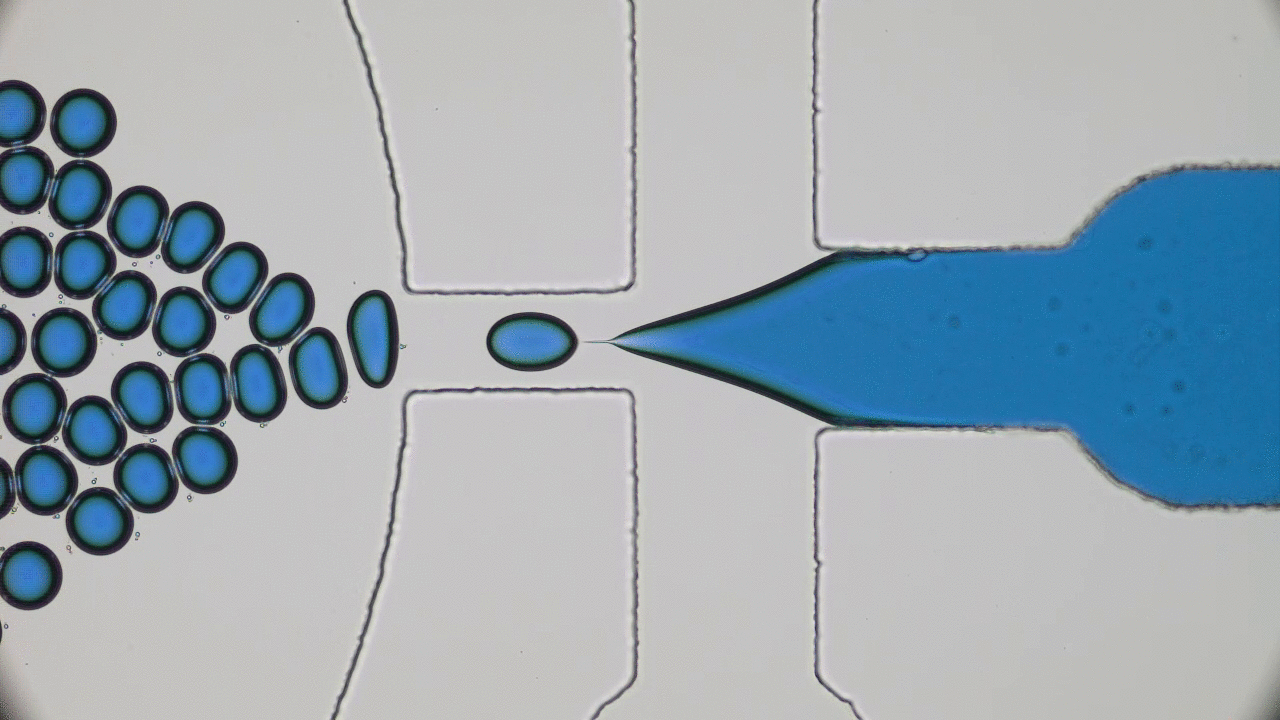
What do a frog’s tongue and a piece of Scotch tape have in common? Not much at first glance. However, if you press your finger on either one of them, you will certainly feel a sensation of stickiness. Indeed, frog saliva acts like a super glue which quickly trap insects. Researchers from Georgia Tech described the saliva biomechanics and highlight some important properties which could be used for high performance adhesive applications
Real soft bites made by a model tongue to better assess food texture
The texture of food products can be tailored so you feel them as soft or hard in your mouth. The measure of food texture is achieved by using tools like a compression machine with two metallic plates to mimic the compression between your tongue and palate. However, the results from these measurements can disagree with the texture we actually perceive because the bottom metallic plate of the machine does not reproduce the deformability of our tongue. This missclassification is especially dangerous for people with swallowing problems who can only eat soft foods. A team of Japanese researchers developed a test machine with a silicone rubber artificial tongue on the bottom metallic plate to better assess food texture.
No pain, no gain : double network hydrogels get stronger after mechanical loading
Pulling too hard on a synthetic soft material like a rubber band usually leads to its failure. However, some biological soft materials, like our muscles, experience the opposite behavior. Our muscles, for instance, grow by repairing damage caused by a mechanical effort, such as lifting weigths. A team of researchers from Hokkaido University successfully mimicked this biological process and applied it to develop hydrogels that get stronger under tensile stress.