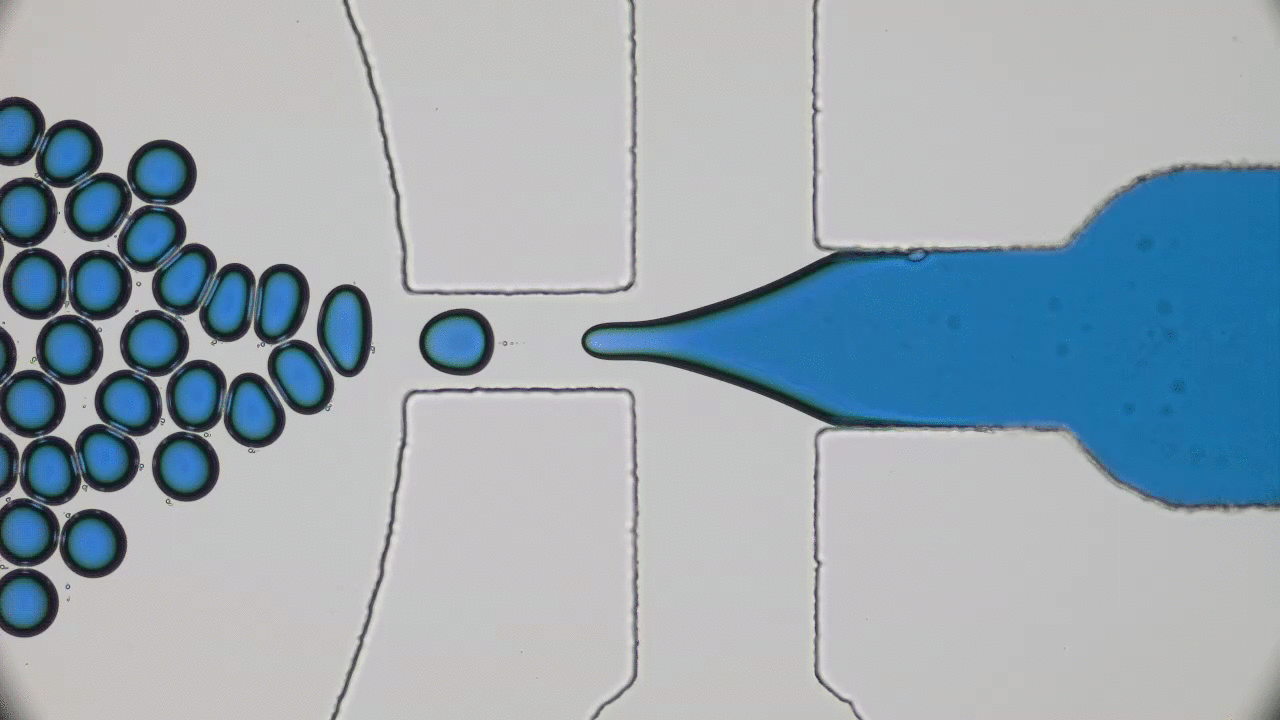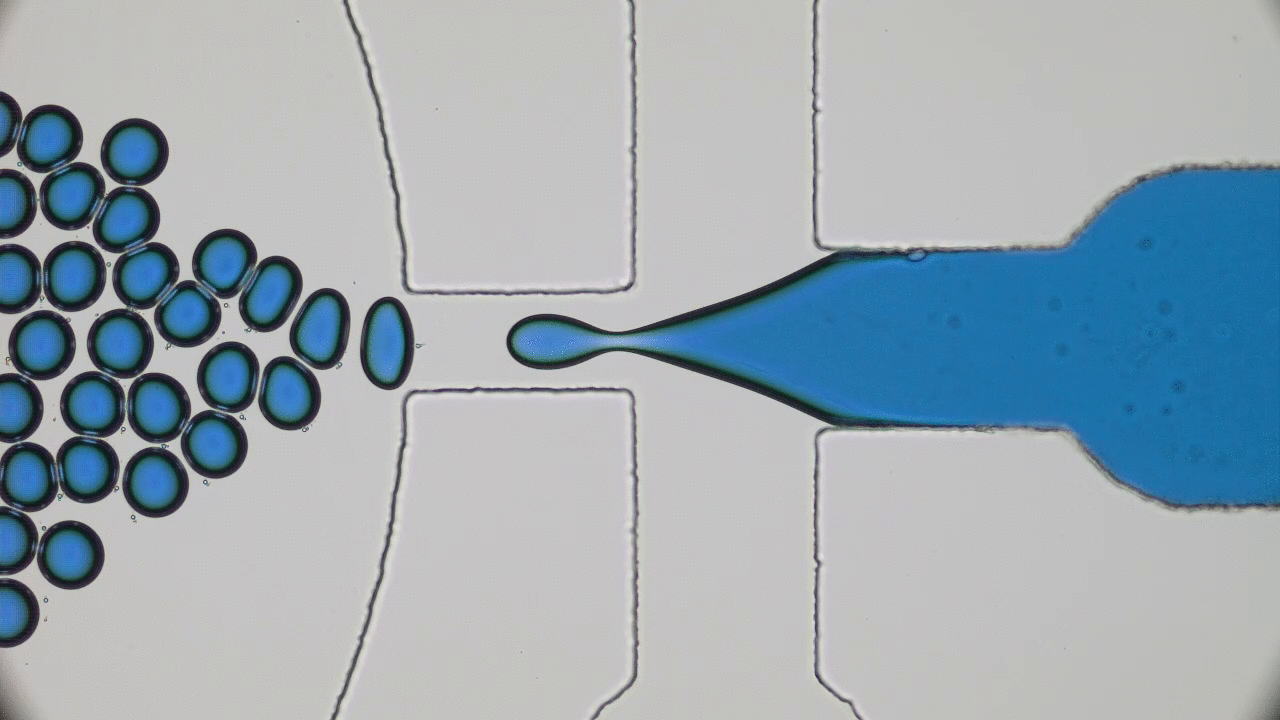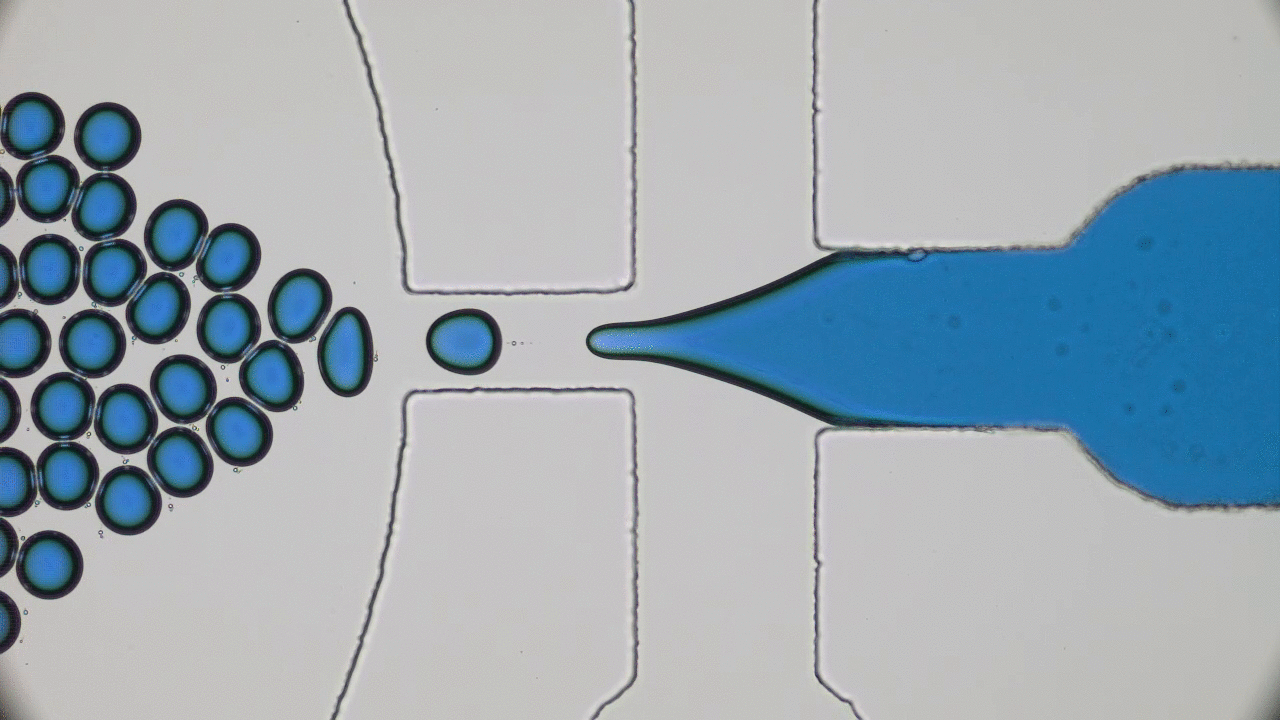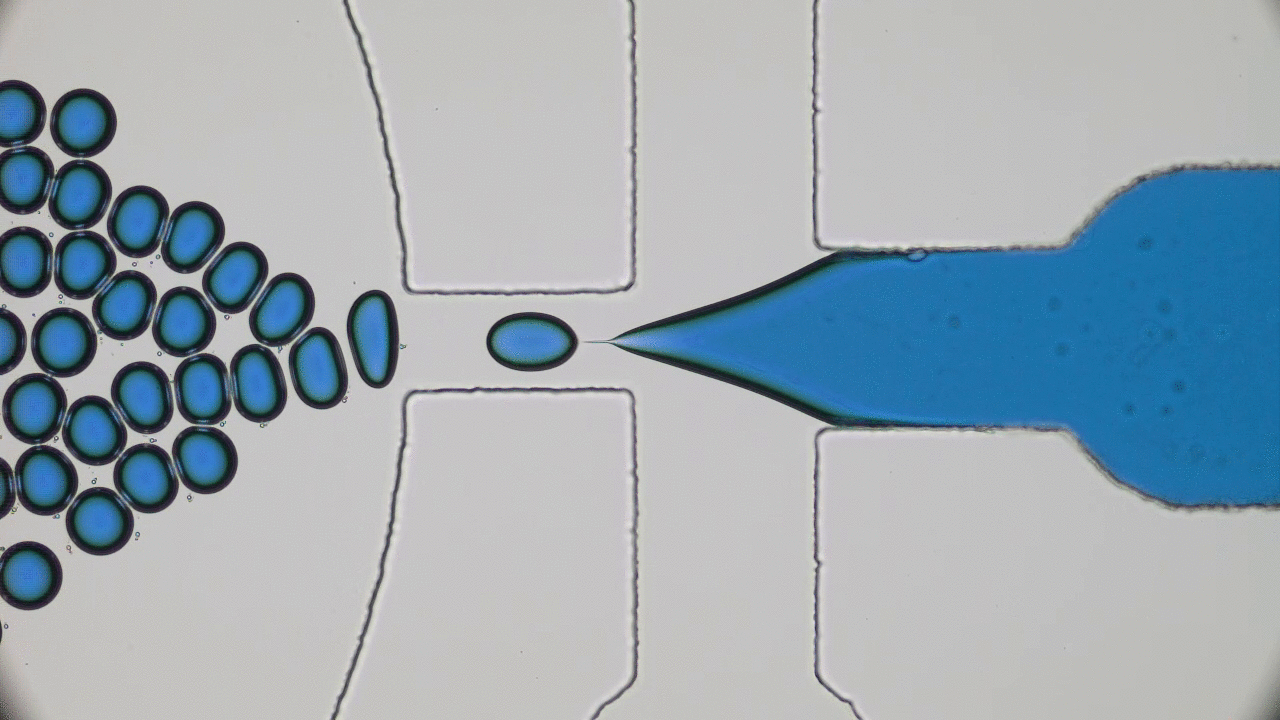
Our bodies rely on many types of tube-shaped organs to transport blood, air, water, food, urine, and feces. These tubular organs are stimuli-responsive: they can constrict or dilate, secrete chemicals, or act as a selective barrier in response to biological signals. Developing synthetic versions of natural tissue structures that mimic biological responses is at the cutting edge of tissue engineering, synthetic organ development, and even soft robotics design. But what materials can be used to grow responsive tubes in the lab?
For the People, By the People: Early career researchers organize virtual polymer physics symposium
In these unprecedented and fluid times, conferences and symposia have gone virtual as STEM collectively settles into a new normal. Many large meetings, like the formerly “in-person only” American Physical Society (APS) and American Chemical Society (ACS) national meetings, have been cancelled or transitioned to virtual-only participation this year. The 2021 Spring APS meeting will go virtual as well. I love big in-person meetings and have shied away from virtual alternatives thinking they would not provide the same feeling of community with my fellow scientists. However, the isolation of quarantine and the desire to get comfortable with the “new normal” motivated me to step out of my comfort zone and into the world of virtual science meetings this summer. So, when the opportunity to attend the 2020 Virtual Polymer Physics Symposium (VPPS) arose in July, I jumped at the chance to participate.