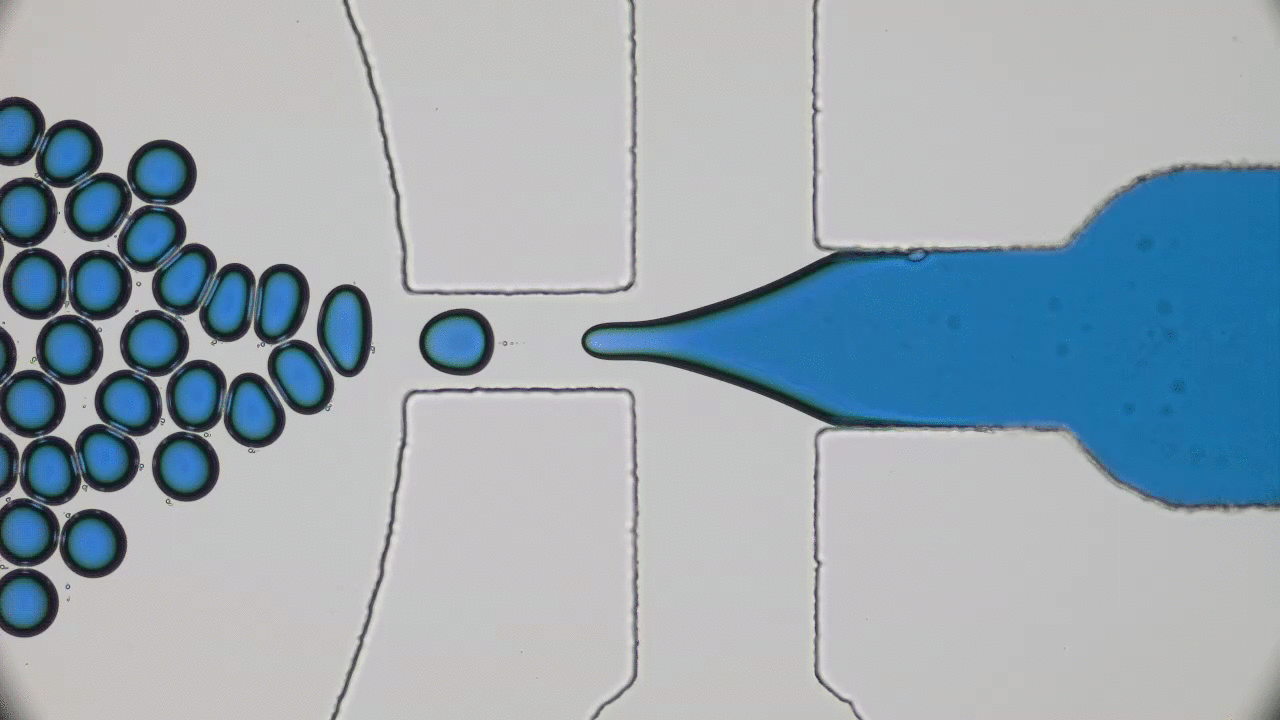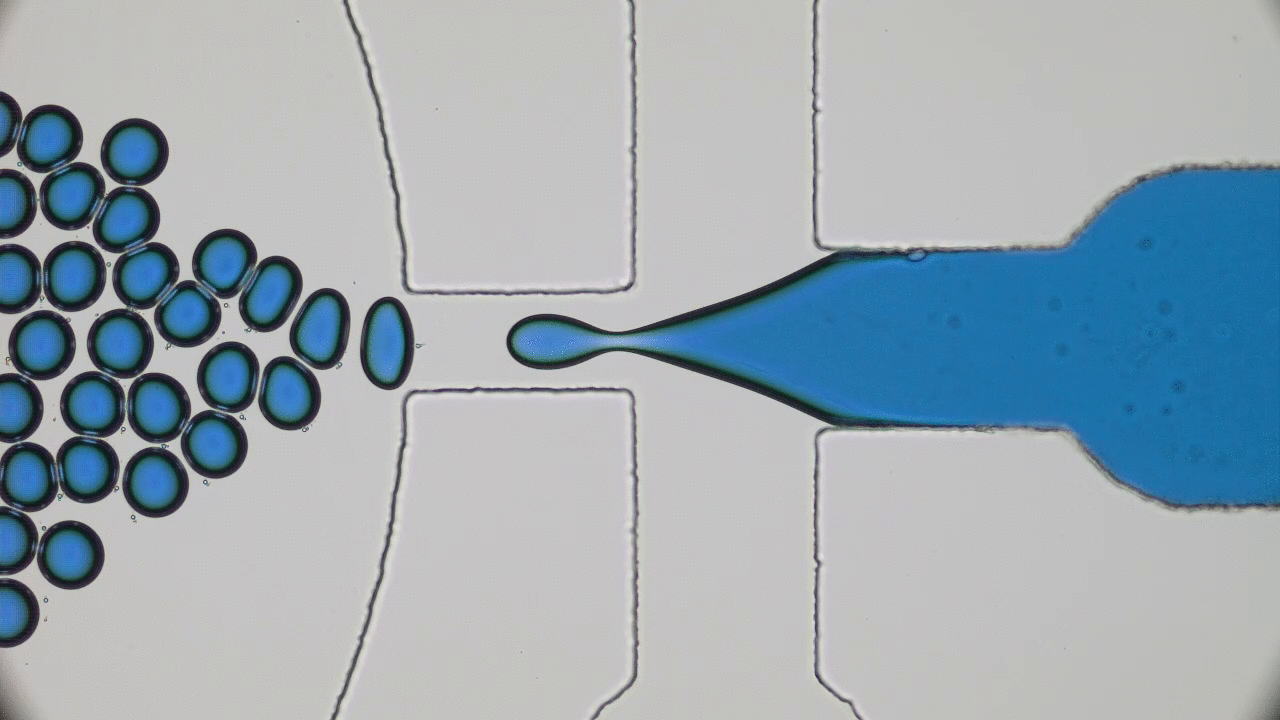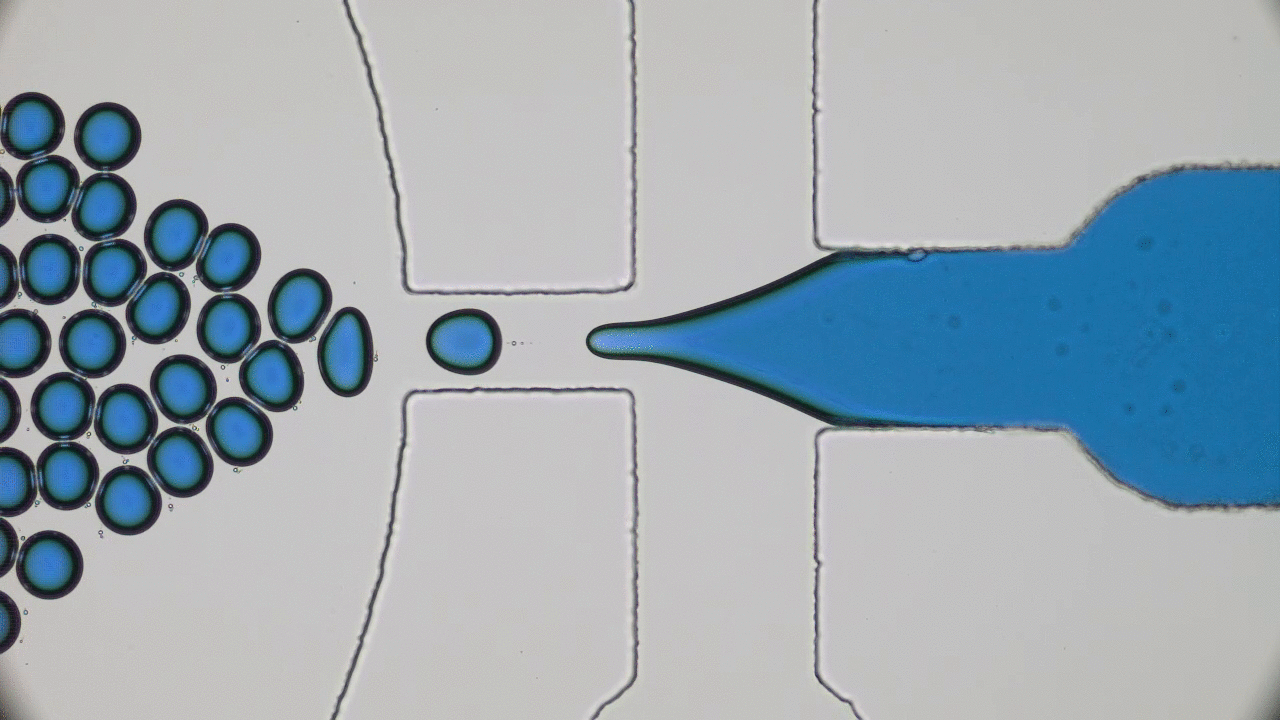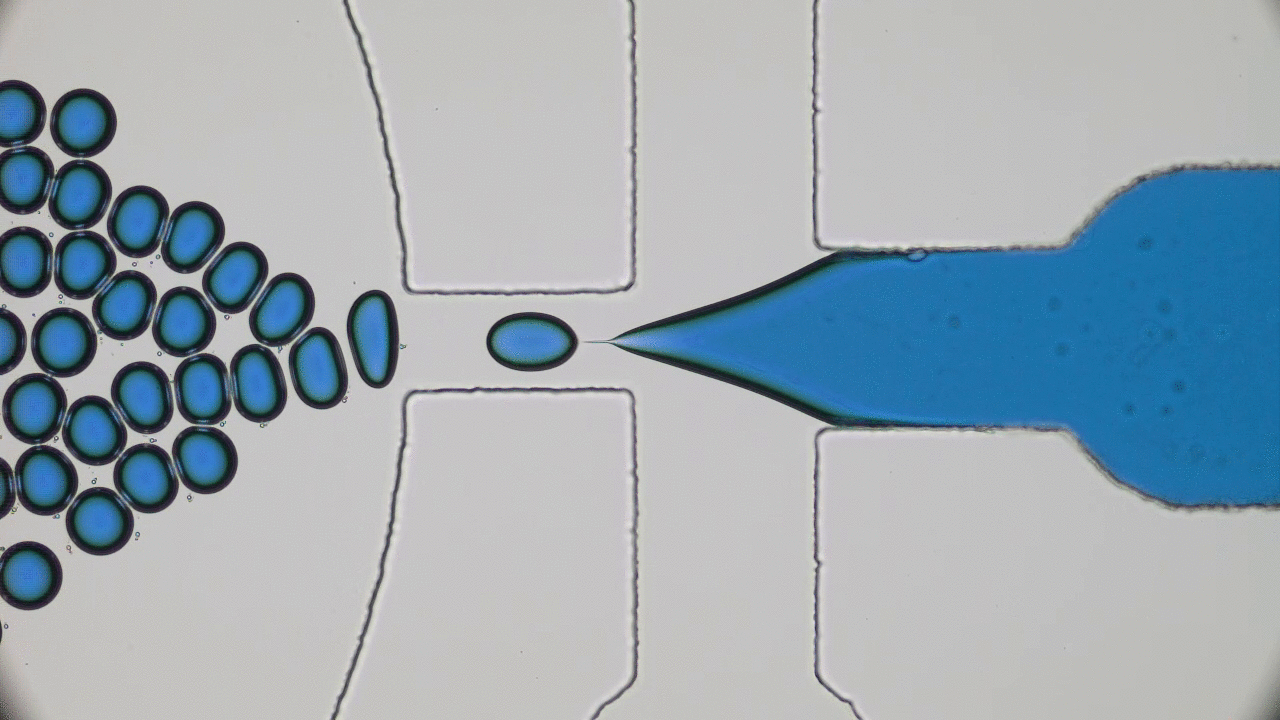
How do squid build a self-assembling “perfect” lens? Research reveals that diffusion and cell biology are the key.
Anti-biofilm Material to Fight Bacterial Formation on Surfaces
Biofilms cause health problems for millions of people worldwide every year, primarily because of infections during surgery or consumption of contaminated packaged foods. To prevent these problems, some scientists are developing surface coatings that will prevent biofilm formation in the first place. In this week’s paper, we will learn about a new technique for creating a microscopic “shield” against the formation and growth of biofilms.
Scientists dream of micro-submarines
In the 1966 movie Fantastic Voyage, a submarine and its crew shrink to the size of a microbe in order to travel into the body of an escaped Soviet scientist and remove a blood clot in his brain. The film gave viewers a glimpse into a possible future where doctors could treat patients by going directly to the source of the problem instead of being limited by the inaccessibility of most parts of the human body. This dream of a tiny submarine that can be piloted through the human body to deliver medical care remains, even 50 years later, in the realm of science fiction. However, Miskin and coworkers at Cornell University have brought us one step closer to making this a reality with their recent development of autonomous microscale machines.
Self-assembling silk lasers
When I first learned about the coffee ring effect I thought it was super cool, but it seemed like an open-and-shut case. Why do rings form where some liquids, like spilled coffee, are left to dry? Roughness on the table causes the liquid to spread imperfectly across the surface, pinning the edges of the droplet in place with a fixed diameter. Because the diameter of the droplet can’t change during evaporation, new liquid must flow from the droplet’s center to the edges. This flow also pushes dissolved coffee particles to the edges of the droplet, where they are left behind to form a ring as the water evaporates away (Figure 1). More details can be found in our previous post, here. It’s a complicated phenomenon, but after being described in 1997 it doesn’t seem like anything new would be going on here. Right? Well, as it usually happens in science, classic concepts have a way of popping back up in unexpected ways. Last year It?r Bak?? Do?ru and her colleagues in Prof. Nizamo?lu’s group at Koç University, Turkey published a study using the often troublesome coffee ring effect to their advantage: making self-assembling silk lasers.
Soft nanoparticles: when polymers meet soap
For more than four decades, scientists have been investigating the properties of small objects dispersed in solutions. Some of these objects – produced in laboratories – are the so called soft nanoparticles. The name soft comes from the fact that these particles are partly solid and partly liquid. One of the scientists’ aims is to design nanoparticles that will be used as carriers of medical compounds (like drugs, DNA segments, and enzymes). The nanoparticles’ role will be to protect this cargo from partial degradation through the human body until reaching the specific target cells where the nanoparticles’ structure will break up and the useful compounds will be released. This technology will allow for disease treatments using smaller amounts of drugs, which will mean fewer side effects for the patients.