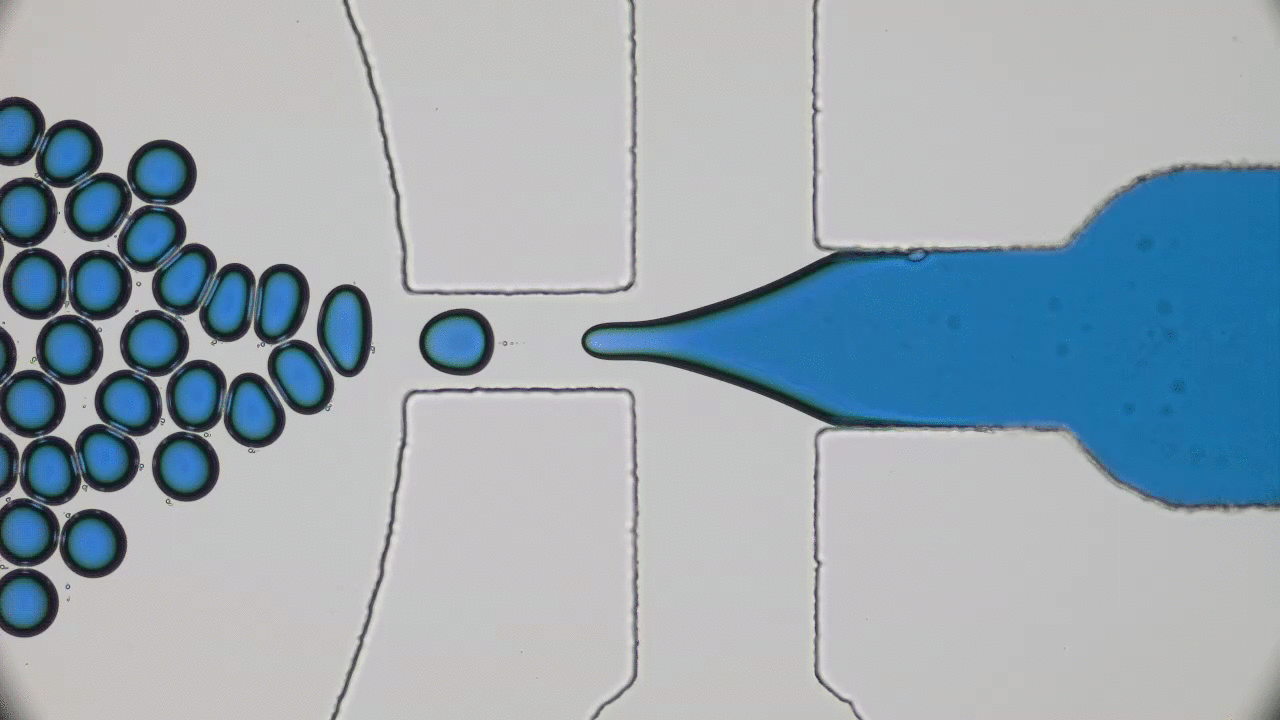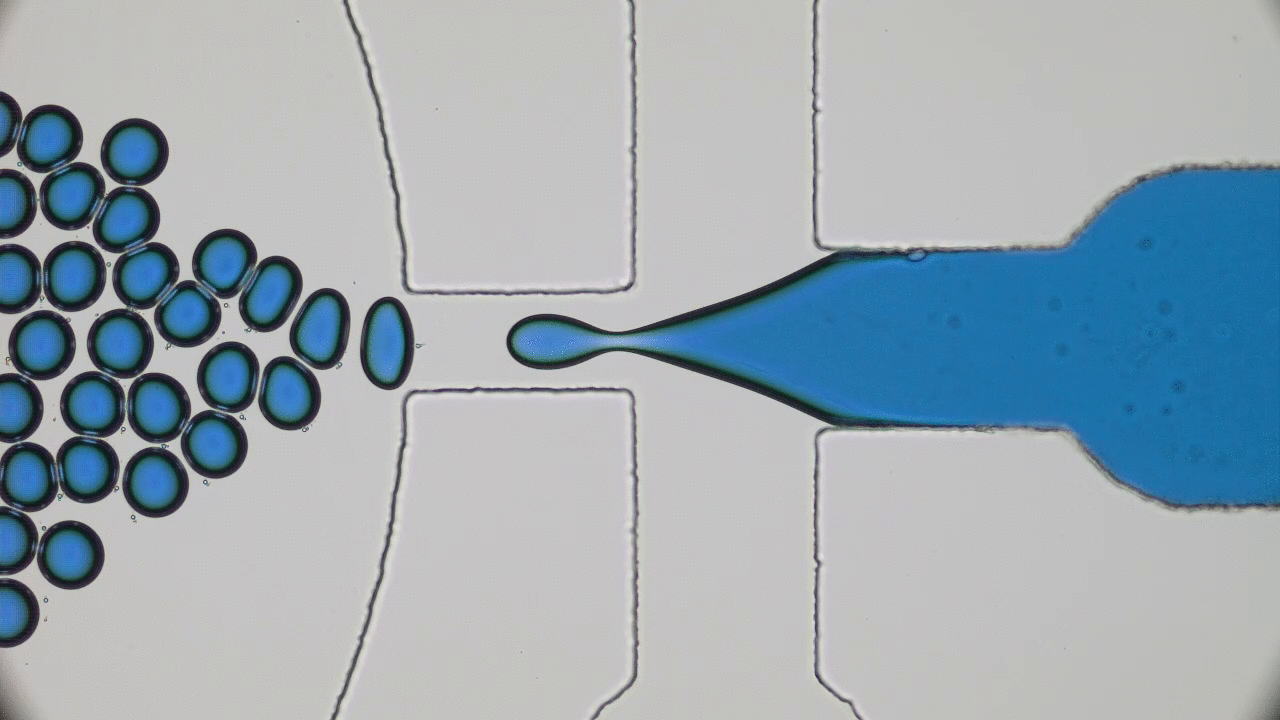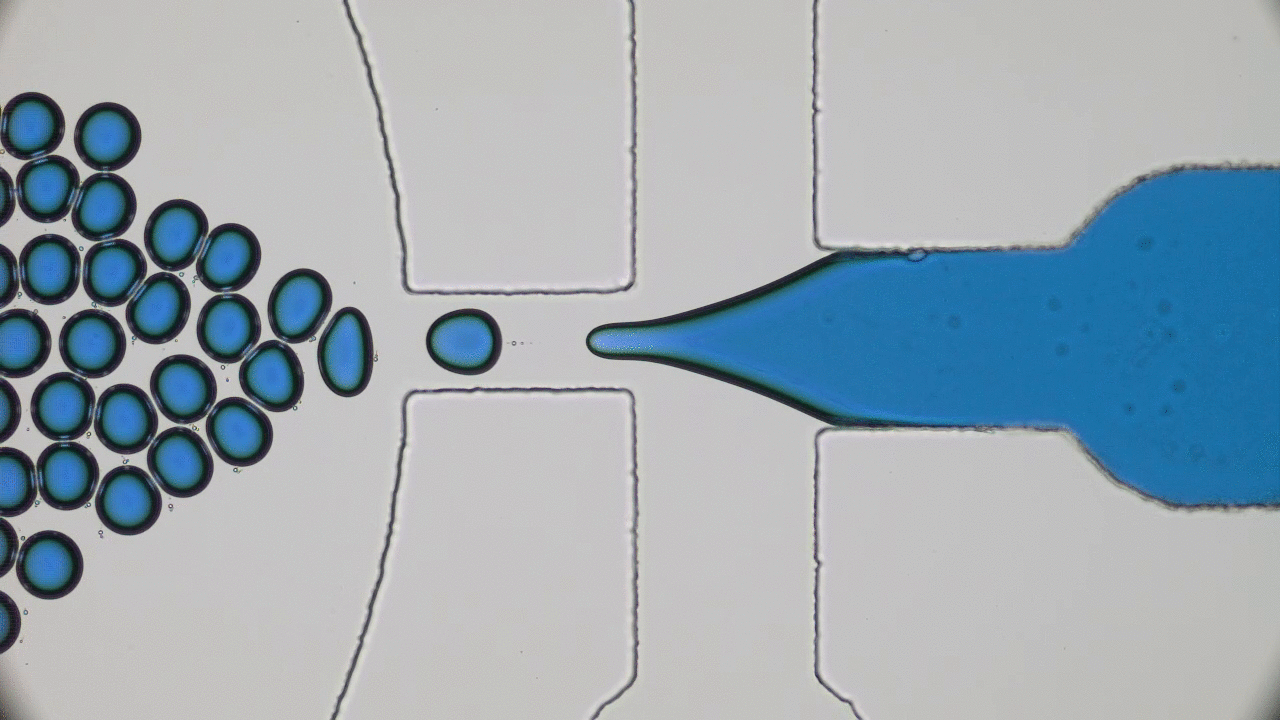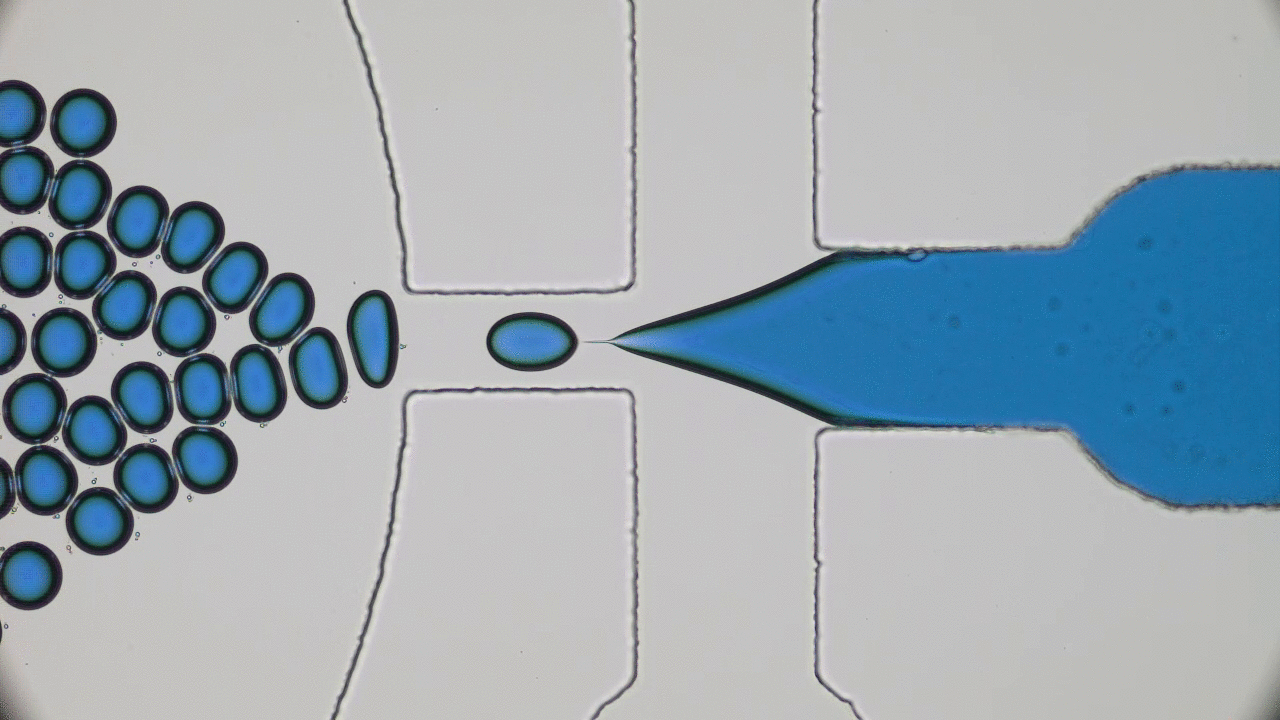
In these unprecedented and fluid times, conferences and symposia have gone virtual as STEM collectively settles into a new normal. Many large meetings, like the formerly “in-person only” American Physical Society (APS) and American Chemical Society (ACS) national meetings, have been cancelled or transitioned to virtual-only participation this year. The 2021 Spring APS meeting will go virtual as well. I love big in-person meetings and have shied away from virtual alternatives thinking they would not provide the same feeling of community with my fellow scientists. However, the isolation of quarantine and the desire to get comfortable with the “new normal” motivated me to step out of my comfort zone and into the world of virtual science meetings this summer. So, when the opportunity to attend the 2020 Virtual Polymer Physics Symposium (VPPS) arose in July, I jumped at the chance to participate.
Slithering Like A Snake and Beyond: Microscopy of Polymer Dynamics
Scientists often draw inspiration from biological organisms to describe phenomena, even when they are studying outside the realm of biology. Physicist Pierre-Gilles de Gennes was no exception. In 1971, after being inspired by the movement of snakes, he proposed reptation theory, or the reptation model, which has since been widely used to describe motions of polymers. As the name “reptation” suggests, de Gennes assumed polymer chains move like snakes. As shown in Figure 1, the model describes a polymer chain’s motion in an environment that is highly populated by other chains (shown in gray) by assuming that the chain is confined in a virtual tube (shown in red) formed by surrounding polymer chains. According to reptation theory, the chain wiggles through this tube, similar to a snake slithering through the woods. As one might imagine, directly imaging the snake-like slithering of polymers is a challenging affair; however, in today’s study, Maram Abadi and coworkers from King Abdullah University of Science and Technology were able to do just that with DNA chains – an example of a polymer – and compared their results to prevailing theory.
“Precise” Polymers Promote Fast Proton Transport
One of the greatest challenges of making proton exchange membrane fuel cells is designing the membrane after which they are named. Engineers would love to have a membrane that transports protons quickly and efficiently. Although there are polymeric materials (e.g. Nafion) that can do this fairly well, there is still a need for faster transport. This week’s paper investigates the role of a polymer’s “precise” structure in facilitating fast proton transport.