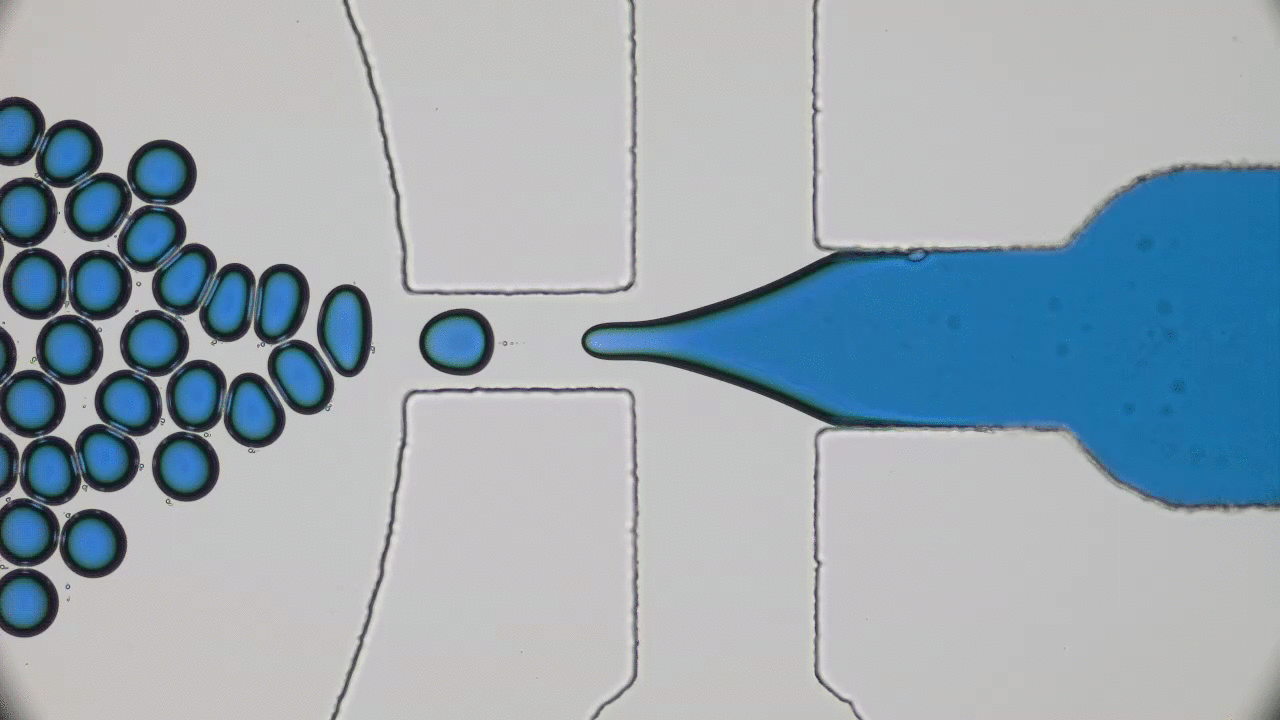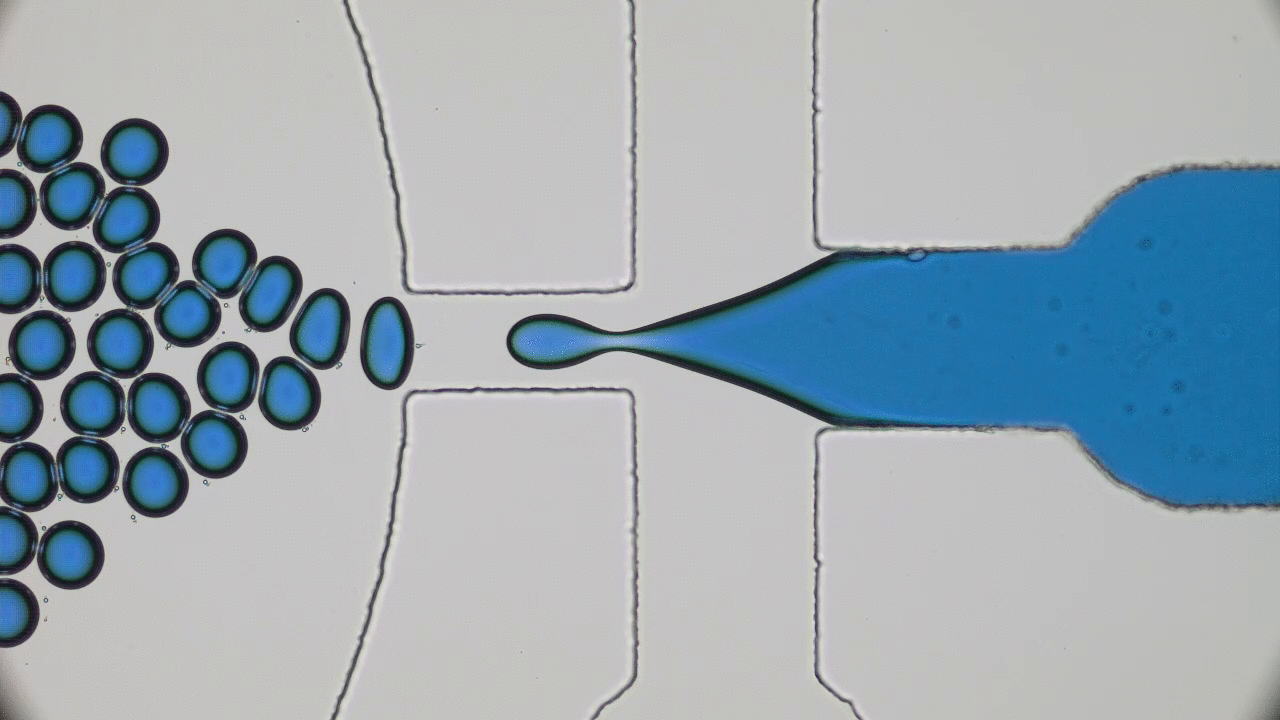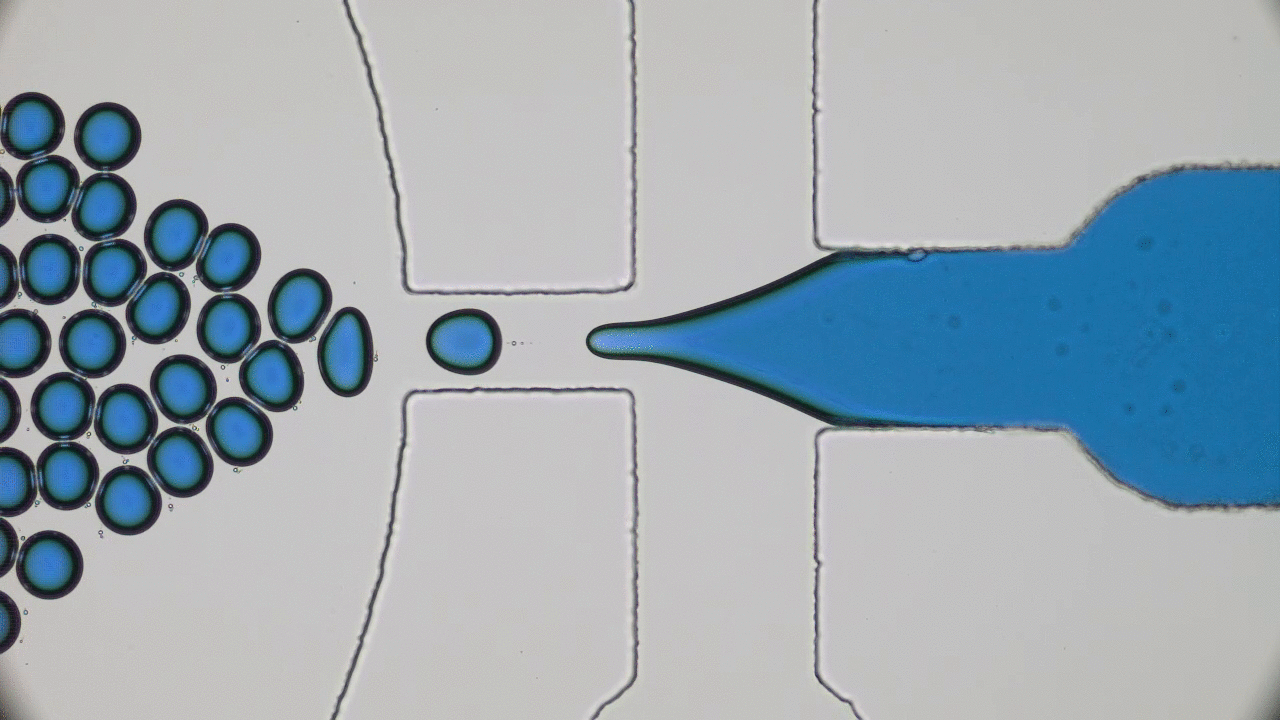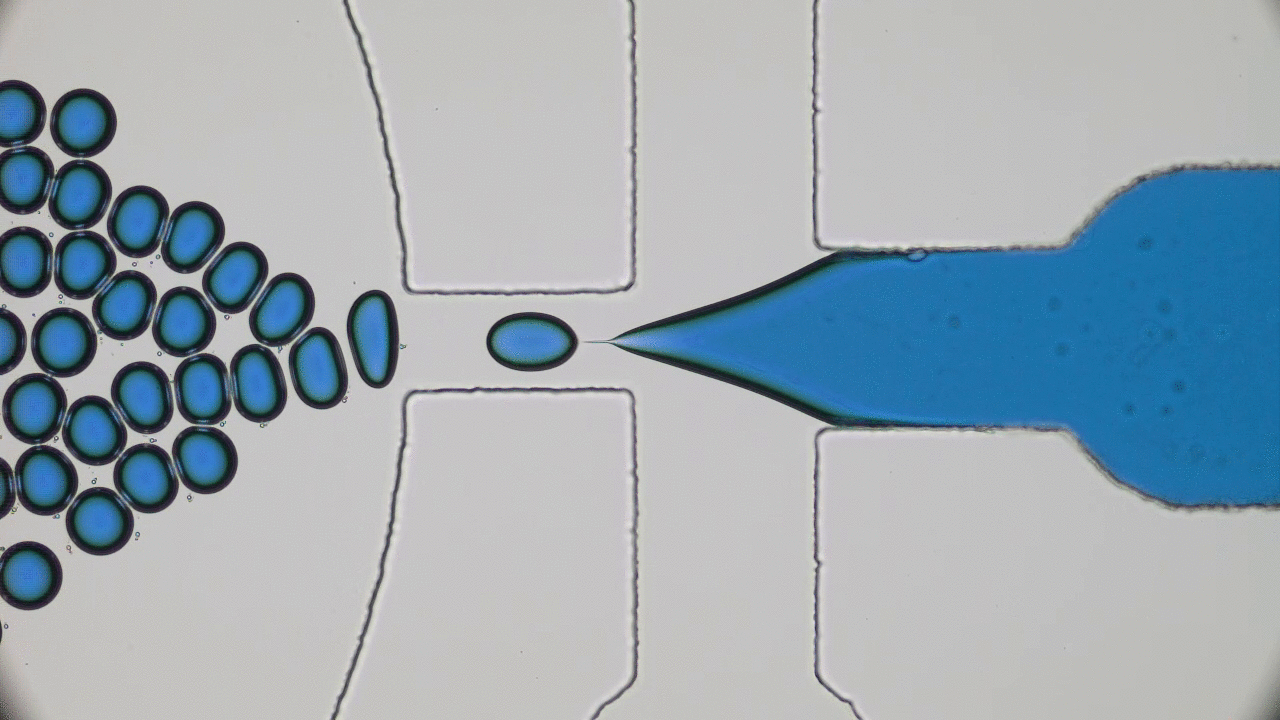
Quintessential soft matter problems, such as the behavior of droplets in ink-jet printing, involve complex interactions between forces and materials. In today’s article, Prof. Wilson Poon points out that coronaviruses are also quintessential soft matter objects, and highlights a range of areas where soft matter science may help better understand, and combat viral pandemics.
When you pull on a drop, how does it pull back?
Physicists like to ignore things. In some cases, we may neglect gravity or assume that the temperature is zero degrees Kelvin — colder than any known substance in the universe. And friction is almost comically absent in most models, despite the fact that a world without it would be utterly uninhabitable (this is nicely illustrated in cartoon form here: https://xkcd.com/669/).
Spider silk: Sticky when wet
If you were Spider-Man, how would you catch your criminals? You could tangle them up in different types of threads, but to really keep them from escaping you probably want your web to be sticky (not to mention the utility of sticky silk for swinging between buildings) …