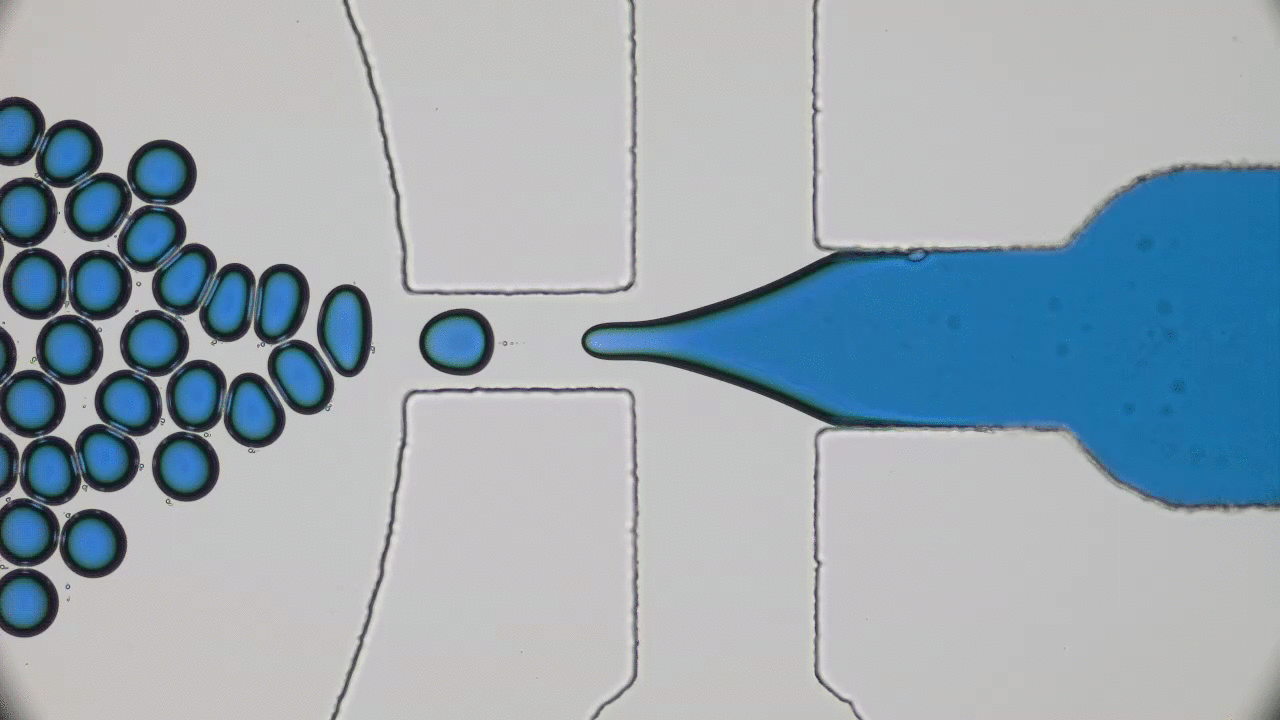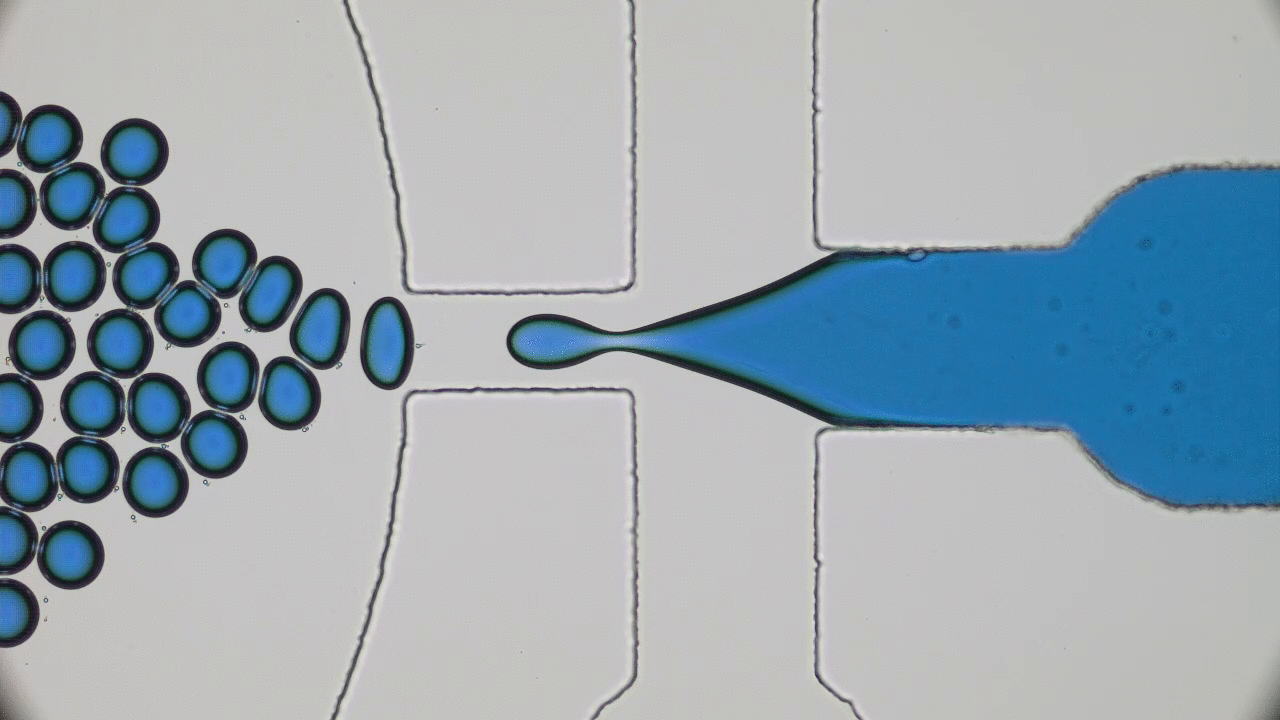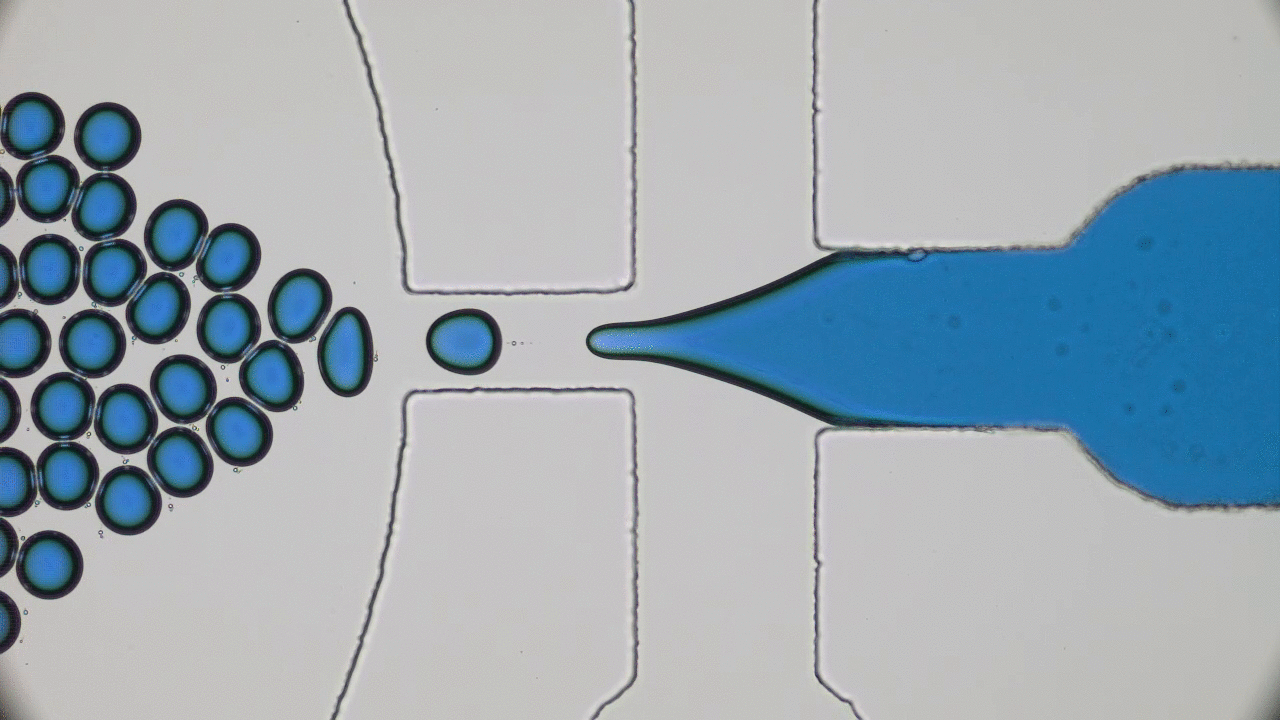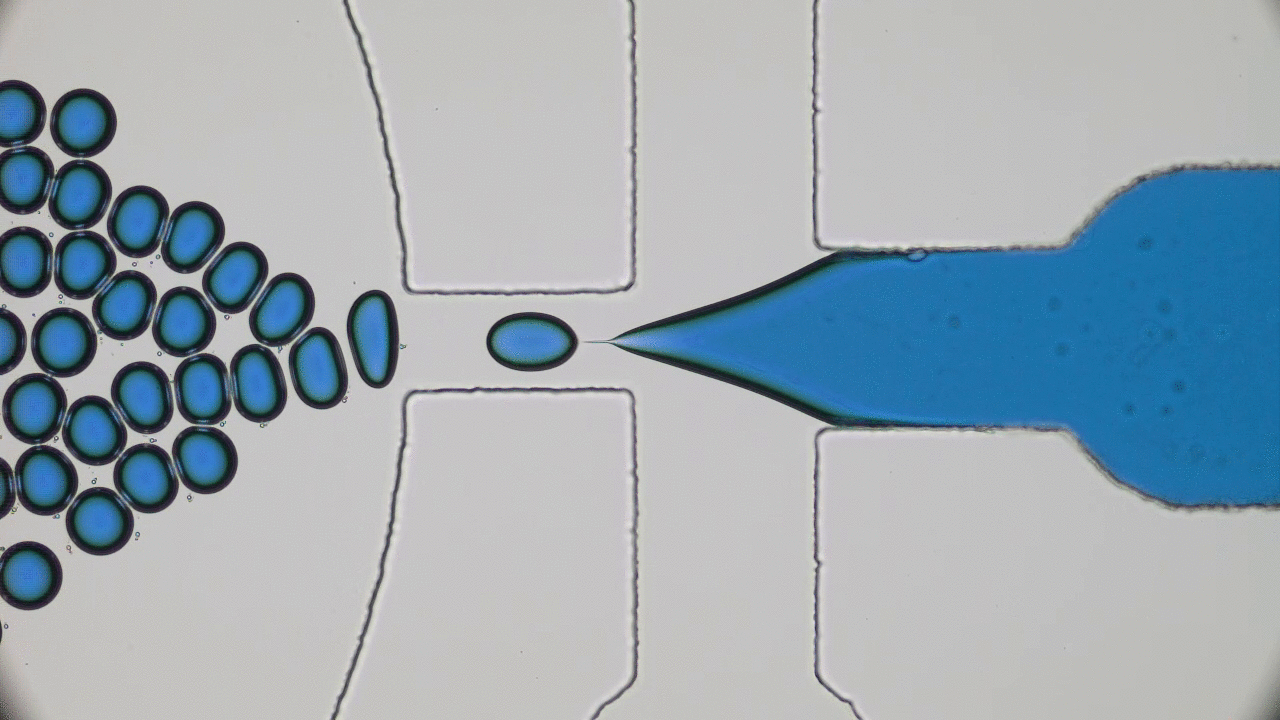
How do squid build a self-assembling “perfect” lens? Research reveals that diffusion and cell biology are the key.
Synthetic blood to power vascularized robots
The circulatory or vascular system of animals is a complex organ that performs multiple functions simultaneously. Take the human circulatory system for example: it transports oxygen and nutrients throughout the body, regulates temperature, helps in fighting diseases, etc. However, robots don’t usually function the same way. Despite the vast improvements in robot design, they still cannot do much multi-tasking. Part of the problem is that robots are typically built from rigid parts that only do one thing. A battery usually does not play any other role aside from providing energy, and moving parts are typically controlled by independent actuators. These single-purpose components (such as batteries) are typically less efficient than their biologic counterparts, and add to the weight of the robots (limiting their performance, maneuverability, speed, and autonomy).
Rebuilding hard matter with soft matter
The skeleton is the backbone of the body, both literally and figuratively. Healthy bones protect soft organs from injury and enable the body to move. Starting from childhood, staying active and following a healthy diet helps the body maintain healthy bones. However, as people age, their bones can start to weaken. There are often no early symptoms to weakening bones, and as a result the first indication of a problem may be a painful break once the weakening has already significantly progressed.