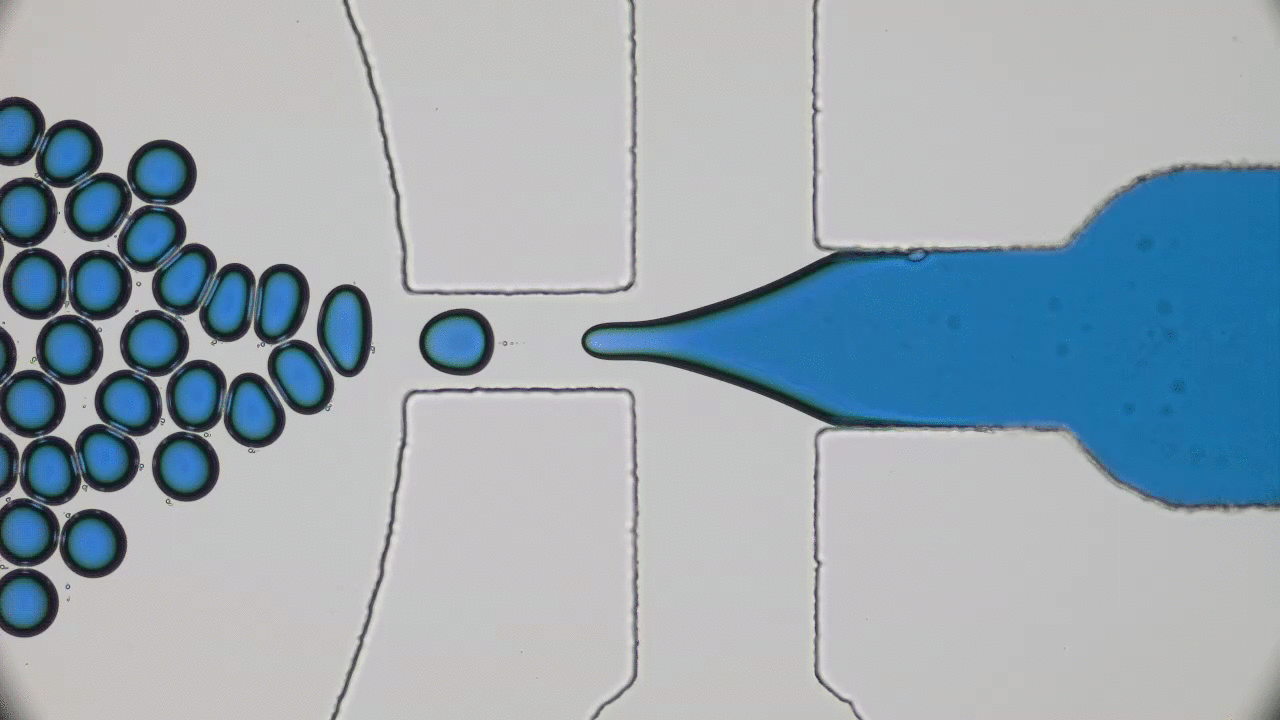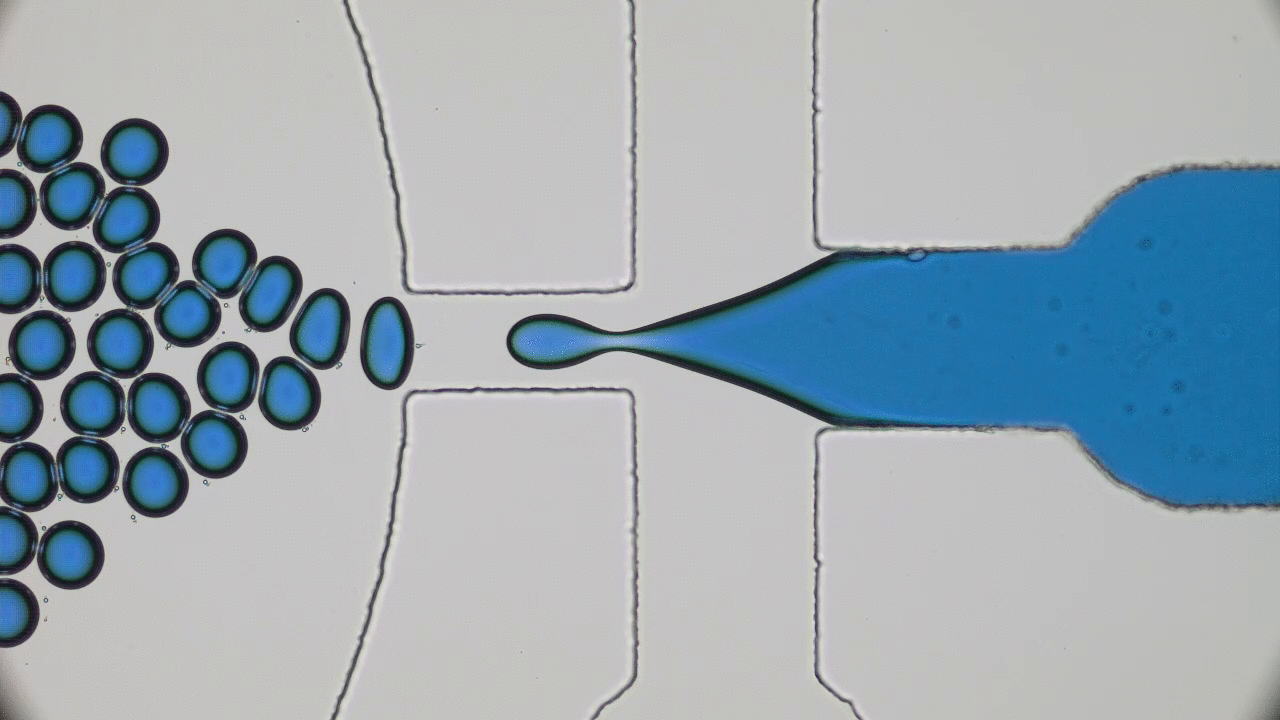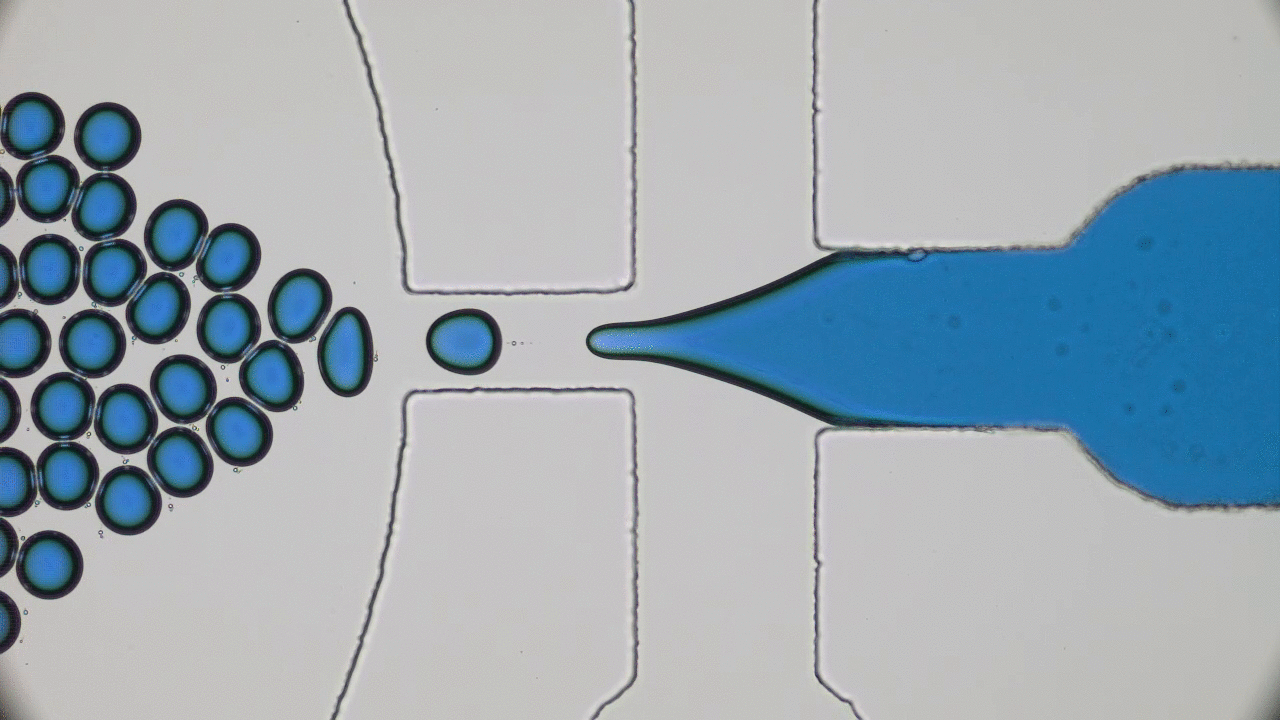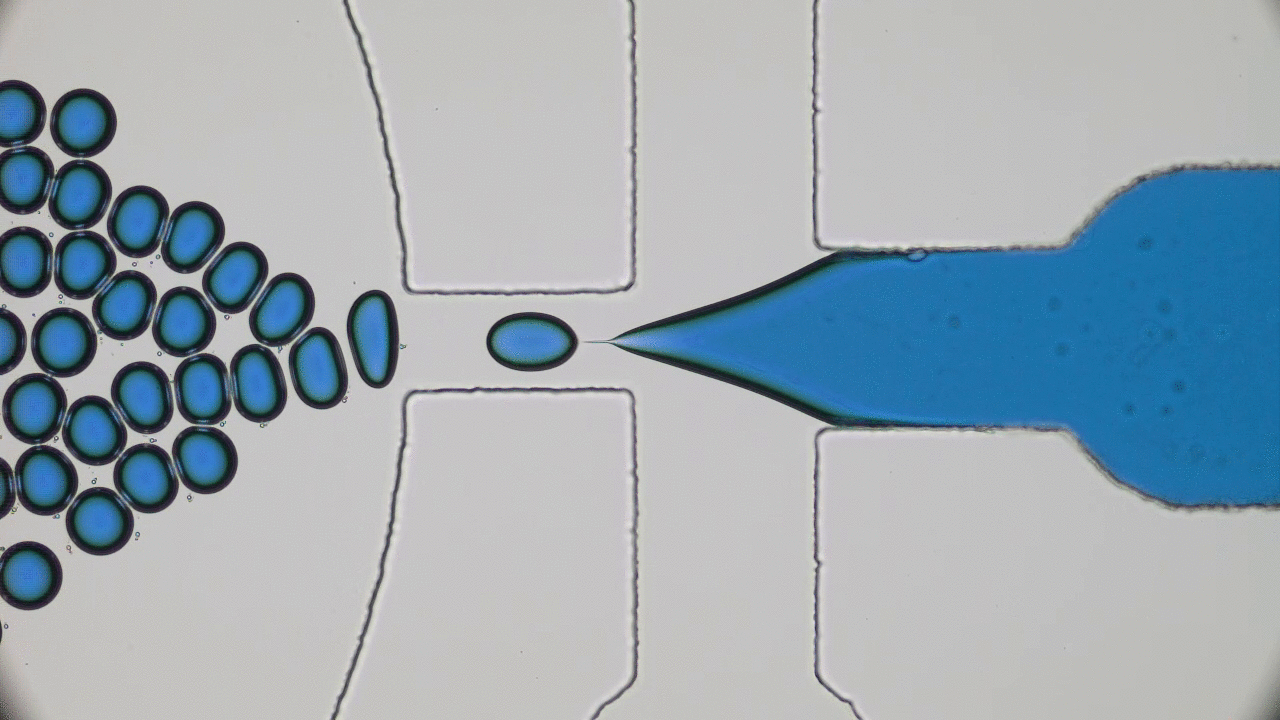
Human skin has many functions beyond ensuring that all of our insides stay, well, inside. Skin also acts as a giant sensor that feels sensations like pressure, temperature, or vibration, and converts them into electrical signals to be processed by the brain. In the animal kingdom, some species like chameleons can even use their skin to selectively blend into their environments. Scientists have set out to create electronic skins, or e-skins, that can mimic or even outperform the typical functions of the human skin by taking on color changing abilities like chameleon skin.
Don’t French kiss the frog prince : how to make better adhesives by mimicking the frog tongue and saliva
What do a frog’s tongue and a piece of Scotch tape have in common? Not much at first glance. However, if you press your finger on either one of them, you will certainly feel a sensation of stickiness. Indeed, frog saliva acts like a super glue which quickly trap insects. Researchers from Georgia Tech described the saliva biomechanics and highlight some important properties which could be used for high performance adhesive applications
Real soft bites made by a model tongue to better assess food texture
The texture of food products can be tailored so you feel them as soft or hard in your mouth. The measure of food texture is achieved by using tools like a compression machine with two metallic plates to mimic the compression between your tongue and palate. However, the results from these measurements can disagree with the texture we actually perceive because the bottom metallic plate of the machine does not reproduce the deformability of our tongue. This missclassification is especially dangerous for people with swallowing problems who can only eat soft foods. A team of Japanese researchers developed a test machine with a silicone rubber artificial tongue on the bottom metallic plate to better assess food texture.
Are squid the key to invisibility?
While many today would associate a “cloak of invisibility” with Harry Potter, the idea of a magical item that renders the wearer invisible is not a new one. In Ancient Greek, Hades was gifted a cap of invisibility in order to overthrow the Titans, whereas in Japanese folklore, Momotar? loots a straw-cloak of invisibility from an ogre, a story which is strangely similar to the English fairytale Jack the Giant-Slayer. Looking to the future in Star Trek, Gene Roddenberry imagined a terrible foe known as the Klingons, a war-driven race that could appear at any moment from behind their cloaking devices – indeed, any modern military would bite your arm off to get hold of this kind of device. Clearly, invisibility is a concept that has captured minds across many cultures, genres, and eras, so it should be no wonder that scientists are working on making it a reality.
Color made from structures inspired by bird feathers
There’s a reason why the word “peacock” has become a verb synonymous with commanding attention. Of course the size of the peacock tail is enough to turn heads, but it wouldn’t be nearly as beautiful without its signature iridescent, or angle-dependent, color. The brilliant colors of the peacock come from the interaction of light with the nanoscale structure of the feathers, which is much different from the origin of color in regular dyes and pigments. In today’s paper, Jason Forster and his colleagues in the Dufresne group developed a simple way to make colors that is inspired by the structures in certain bird feathers. To understand how it works, let’s start from the beginning.
Fluids That Flow Themselves
When we think about fluid flow, we generally think of motion in response to some external force: rivers run downhill because of gravity, while soda moves through a straw because of the pressure difference created by sucking on one end. Recently, however, scientists have become interested in a class of fluids that have the capacity to move all by themselves — the so-called “active fluids.” In this paper, Kun-Ta Wu and his co-workers explore how such a material can turn its stored chemical energy into useful work: cargo transport.