Original paper: Statistical Physics of Self-Replication
Content review: Adam Fortais
Style review: Andrew Ton
Understanding the origin of life is one of the most enduring and fundamental scientific challenges there is. Of all branches of science, physics is probably not the first place one would think to go to for enlightenment. Life seems too complicated and multi-layered to be captured by the simplistic frameworks of physics. Today’s paper tackles a small part of understanding the origin of life – the physics of self-replication.
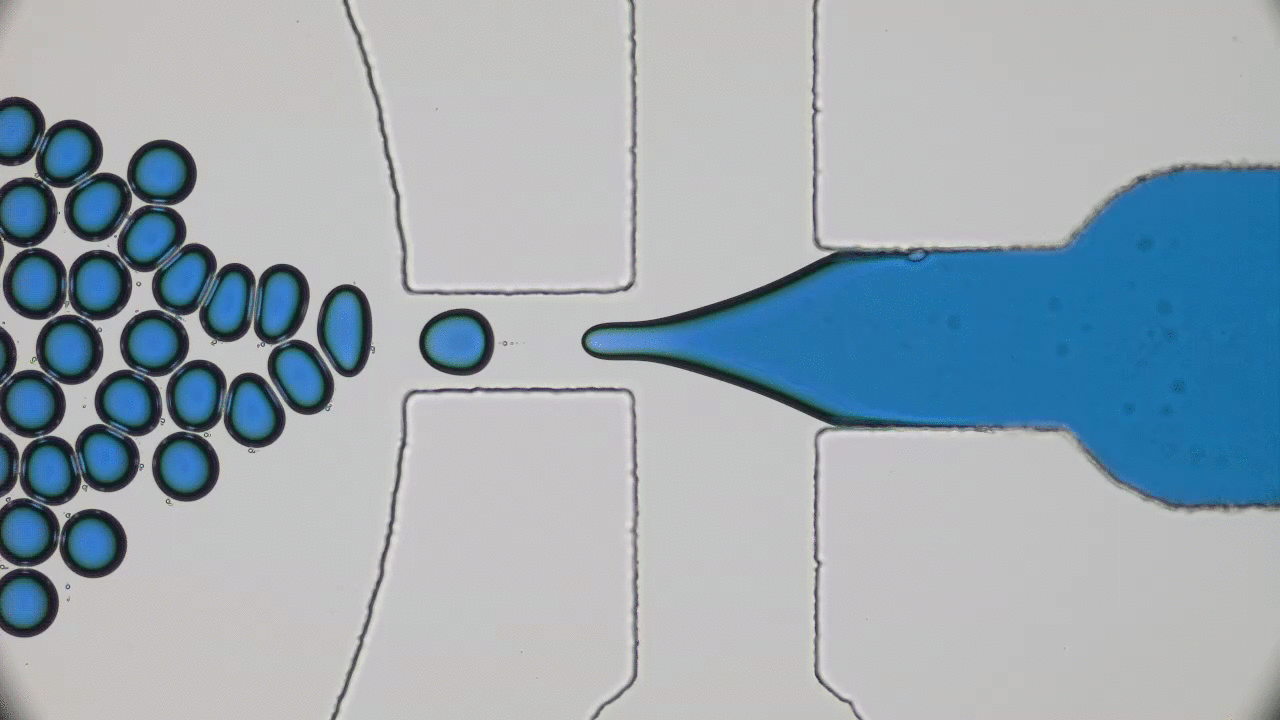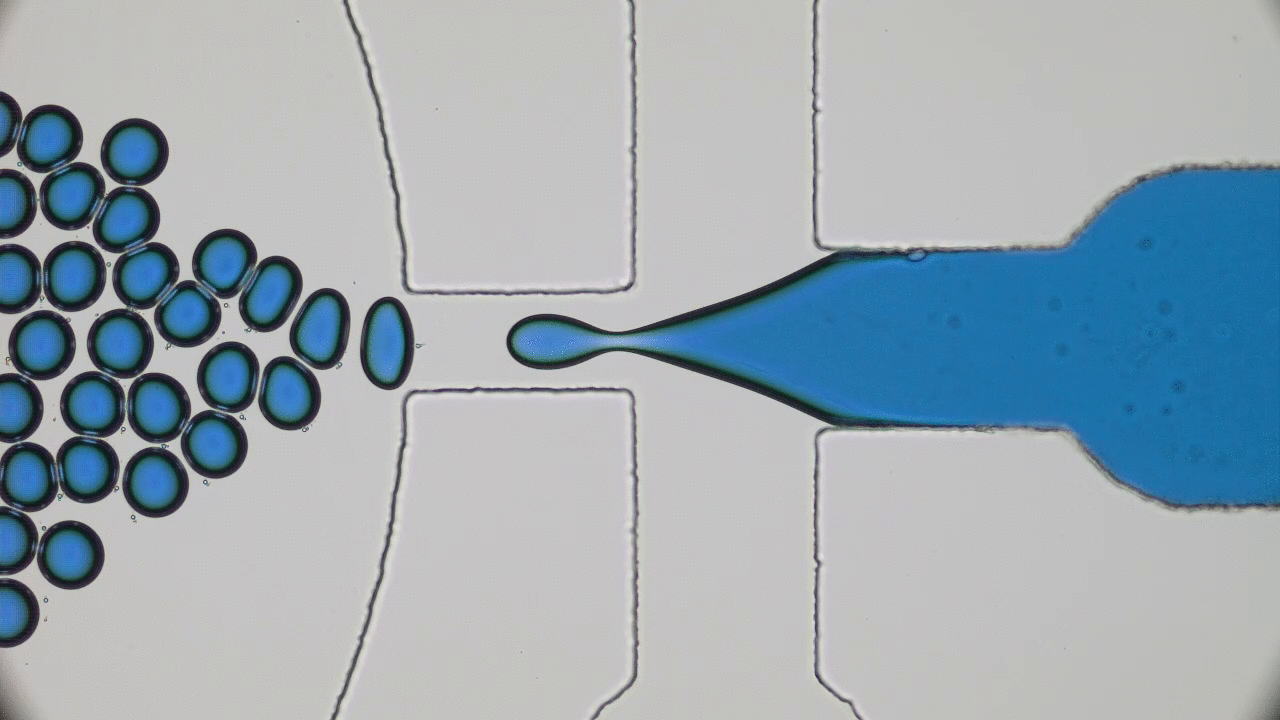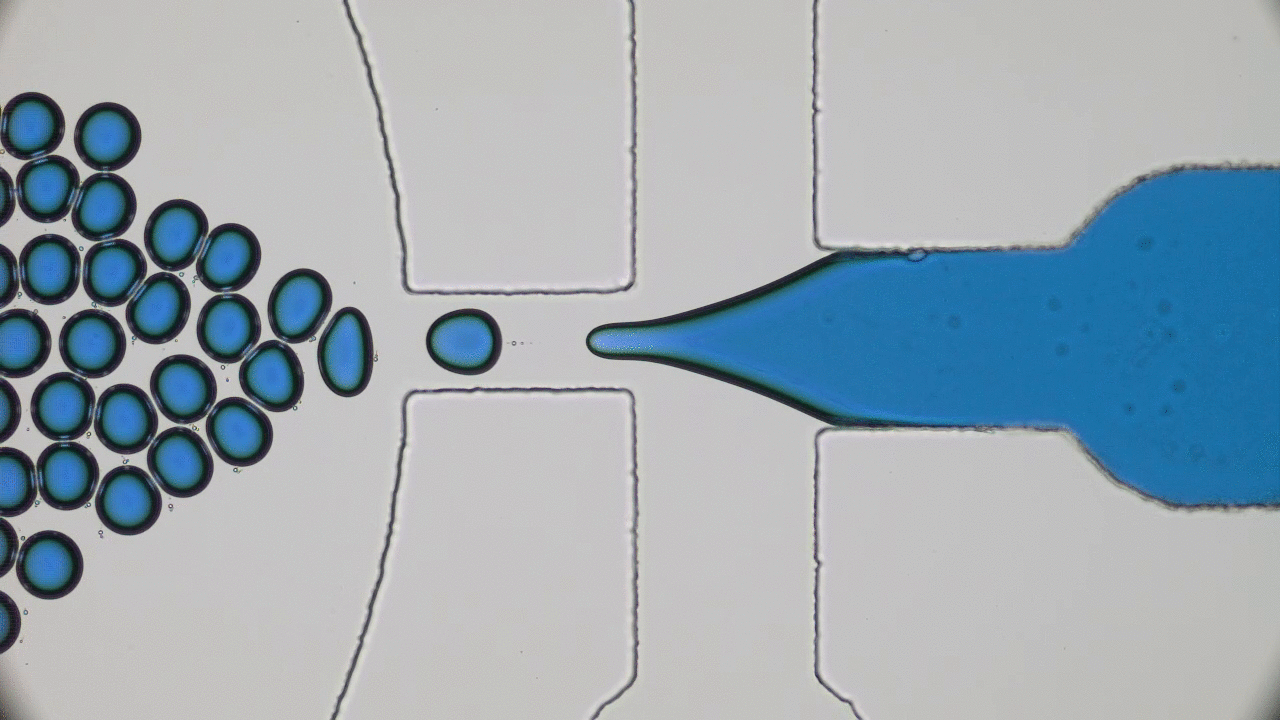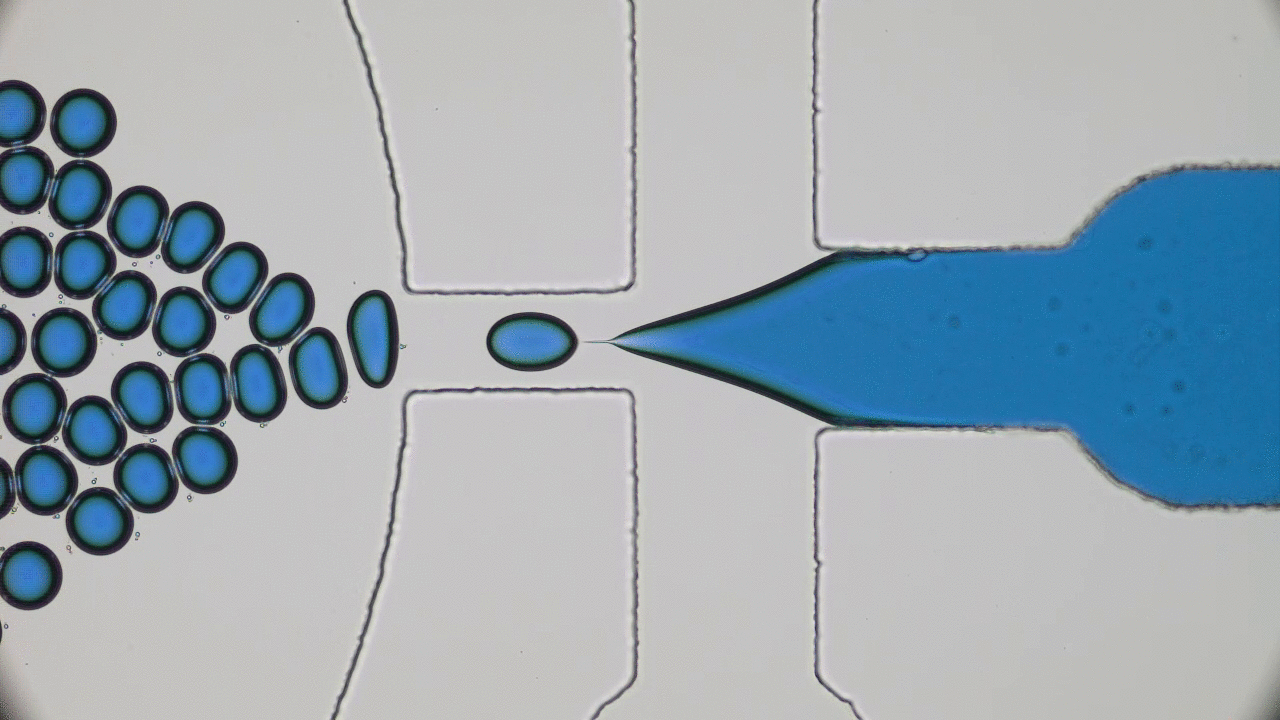
This paper begins by considering two macroscopic states, shown in Figure 1A, which are “one bacterium in a petri dish” and “two bacteria in a petri dish”, and considers transitions between these states. The biggest challenge here is relating a macroscopic change — the replication of a cell or collection of complex molecules — to a set of microscopic operations, also known as chemical reactions. But these reactions are tremendously complicated. How can we know what to expect from them? The answer lies within the art of thermodynamics.

According to thermodynamics, the governing quantities in a typical chemical reaction are energy, heat, and entropy. During a reaction, they can gain or lose any of these three as long as the total energy is kept balanced. Entropy, however, is a bit more special than the other two. Roughly speaking, entropy is a way of counting the number of possible ways a system can be in a certain state. So if a reaction involves several small molecules binding together to form a larger molecule, that involves a big loss in entropy. This is because there are many more ways to organize a large number of molecules than ways to organize one. Thermodynamics tells us that heat must be released to “pay for” this change in entropy. This heat flow increases the entropy of the environment, leading to an overall increase. All other things being equal, systems tend towards states of high entropy, simply because there are more ways of being in those states. This is usually referred to as the Second Law of Thermodynamics.
These are abstract descriptions of thermodynamic processes — how does the author use these to construct more concrete, quantitative models? First, they derive a version of the Second Law which relates the heat released by the transition to the irreversibility of the transition: the harder it is to undo a process, i.e. the more irreversible it is, the more heat must be released. Combining this observation with a simple model of replication, England reaches an important result: for a self-replicating system, the more efficiently it uses the available energy, the more rapidly it will replicate.
England uses thermodynamics as a set of rules to calculate whether cell division for a bacterium is physically possible. While we already know the answer, the author is seeking to understand if this simple theory contains enough details to make accurate estimates about bacterial replication. The hard part of this problem isn’t to calculate the heat or entropy released, but rather to put a physical constraint on the likelihood of the reverse process. After all, we don’t ever see bacteria spontaneously dissolve back into their constituents. But with some clever thinking, this problem can be circumvented. Instead of considering the probability of a bacterium dissolving, the author simply considers the probability of every single chemical bond inside it spontaneously breaking. This is an extremely unlikely event, and yet it’s not as unlikely as the cell spontaneously being unmade, as shown in Figure 1B, and so it can give us a lower bound for the irreversibility of cell division. Combined with careful estimates of heat and entropy transfers, this gives a full (and very approximate) thermodynamic accounting of the process of cell division.
What can we do with this? We can perform some comparisons: first of all, the irreversibility of a process turns out to be a much larger thermodynamic barrier than the entropic difficulty of organizing all the constituents of a daughter bacterial cell, which is a highly structured object! This is surprising at first, but hindsight is 20/20: living systems are doing a lot of work to make things that don’t dissolve back into water. Another surprising conclusion of this argument is that real bacteria are tremendously efficient! With the coarse estimates used here, the author gets a replication rate close to that of a real E. Coli bacterium. This is an astonishing result, since the process considered here is not nearly as irreversible as that of a real cell division.
The takeaway here isn’t simply learning something about bacteria or replication. The real lesson is about the power of the methods of statistical physics. The division of a bacterium is frighteningly complicated, and no physicist could write down the chain of reactions necessary for the proper replication and division of this complex system. Despite this intricacy, biological processes must still follow the unambiguous laws of physics. And that implies one thing: more life, more complexity, and more entropy. While this is by no means an answer to the question “where does life come from?”, it gives us hope that physics will continue to play an important role in the story of answering this question.